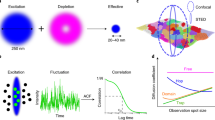
Abstract
Fluorescence microscopy, especially confocal microscopy, has revolutionized the field of biological imaging. Breaking the optical diffraction barrier of conventional light microscopy, through the advent of super-resolution microscopy, has ushered in the potential for a second revolution through unprecedented insight into nanoscale structure and dynamics in biological systems. Stimulated emission depletion (STED) microscopy is one such super-resolution microscopy technique which provides real-time enhanced-resolution imaging capabilities. In addition, it can be easily integrated with well-established fluorescence-based techniques such as fluorescence correlation spectroscopy (FCS) in order to capture the structure of cellular membranes at the nanoscale with high temporal resolution. In this review, we discuss the theory of STED and different modalities of operation in order to achieve the best resolution. Various applications of this technique in cell imaging, especially that of neuronal cell imaging, are discussed as well as examples of application of STED imaging in unravelling structure formation on biological membranes. Finally, we have discussed examples from some of our recent studies on nanoscale structure and dynamics of lipids in model membranes, due to interaction with proteins, as revealed by combination of STED and FCS techniques.










Similar content being viewed by others
References
Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, LippincottSchwartz J and Hess HF 2006 Imaging intracellular fluorescent proteins at nanometer resolution. Science 313 1642–1645
Betzig E, Trautman J, Harris T, Weiner J and Kostelak R 1991 Breaking the diffraction barrier: optical microscopy on a nanometric scale. Science 251 1468–1470
Bianchini P, Cardarelli F, Di Luca M, Diaspro A and Bizzarri R 2014 Nanoscale protein diffusion by STED-based pair correlation analysis. PloS One 9 e99619
Bianchini P, Peres C, Oneto M, Galiani S, Vicidomini G and Diaspro A 2015 Sted nanoscopy: a glimpse into the future. Cell Tissue Res 360 143–150
Blom H and Bates M 2015 Nanoscopyimaging life at the nanoscale: a Nobel Prize achievement with a bright future. Phys. Scr. 90 108010
Boettiger AN, Bintu B, Moffitt JR, Wang S, Beliveau BJ, Fudenberg G, Imakaev M, Mirny LA, Wu C-t and Zhuang X 2016 Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature 529 418–422
Brown DA and London E 2000 Structure and function of sphingolipid-and cholesterol-rich membrane rafts. J. Biol. Chem. 275 17221–17224
Buckers J, Wildanger D, Vicidomini G, Kastrup L and Hell SW 2011 Simultaneous multi-lifetime multi-color STED imaging for colocalization analyses. Opt. Express 19 3130–3143
Cambi A and Lidke DS 2014 Cell membrane nanodomains: From biochemistry to nanoscopy (CRC Press)
Clausen MP, Sezgin E, de la Serna JB, Waithe D, Lagerholm BC and Eggeling C 2015 A straightforward approach for gated STED-FCS to investigate lipid membrane dynamics. Methods 88 67–75
Cossart P and Lebreton A 2014 A trip in the new microbiology with the bacterial pathogen listeria monocytogenes. FEBS letters 588 2437–2445
Cossart P, Vicente MF, Mengaud J, Baquero F, Perez-Diaz J and Berche P 1989 Listeriolysin o is essential for virulence of listeria monocytogenes: direct evidence obtained by gene complementation. Infect. Immun. 57 3629–3636
Dietrich C, Bagatolli L, Volovyk Z, Thompson N, Levi M, Jacobson K and Gratton E 2001 Lipid rafts reconstituted in model membranes. Biophys. J. 80 1417–1428
DEste E, Kamin D, Gottfert F, El-Hady A and Hell SW 2015 Sted nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep. 10 1246–1251
Edidin M 2001 Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol 11 492–496
Eggeling C 2012 Sted-FCS nanoscopy of membrane dynamics; in Fluorescent methods to study biological membranes (Springer) pp 291–309.
Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schonle A, et al. 2009 Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457 1159–1162
Erdmann RS, Takakura H, Thompson AD, Rivera-Molina F, Allgeyer ES, Bewersdorf J, Toomre D and Schepartz A 2014 Super-resolution imaging of the Golgi in live cells with a bioorthogonal ceramide probe. Angew. Chem. Int. Ed. 53 10242–10246
Favard C, Wenger J, Lenne P-F and Rigneault H 2011 FCS diffusion laws in two-phase lipid membranes: determination of domain mean size by experiments and Monte Carlo simulations. Biophys. J. 100 1242–1251
Galbraith CG and Galbraith JA 2011 Super-resolution microscopy at a glance. J. Cell. Sci. 124 1607–1611
Gottfert F, Wurm CA, Mueller V, Berning S, Cordes VC, Honigmann A and Hell SW 2013 Coaligned dual-channel STED nanoscopy and molecular diffusion analysis at 20 nm resolution. Biophys. J. 105 L01–L03
Harke B, Keller J, Ullal CK, Westphal V, Schonle A and Hell SW 2008 Resolution scaling in STED microscopy. Opt. Express 16 4154–4162
Hedde PN, Dorlich RM, Blomley R, Gradl D, Oppong E, Cato AC and Nienhaus GU 2013 Stimulated emission depletion-based raster image correlation spectroscopy reveals biomolecular dynamics in live cells. Nat. Commun. 4 2093
Hein B, Willig KI and Hell SW 2008 Stimulated emission depletion (STED) nanoscopy of a fluorescent protein-labeled organelle inside a living cell. Proc. Natl. Acad. Sci. USA 105 14271–14276
Hell SW 2007 Far-field optical nanoscopy. Science 316 1153–1158
Hell SW 2015 Nanoscopy with focused light (Nobel lecture) Angew. Chem. Int. Ed. 54 8054–8066
Hell SW, Sahl SJ, Bates M, Zhuang X, Heintzmann R, Booth MJ, Bewersdorf J, Shtengel G, Hess H, Tinnefeld P, et al. 2015 The 2015 super-resolution microscopy roadmap. J. Phys. D. 48 443001
Honigmann A, Mueller V, Ta H, Schoenle A, Sezgin E, Hell SW and Eggeling C 2014a Scanning STED-FCS reveals spatiotemporal heterogeneity of lipid interaction in the plasma membrane of living cells. Nat. Commun. 5 6412
Honigmann A, Sadeghi S, Keller J, Hell SW, Eggeling C and Vink R 2014b A lipid bound actin meshwork organizes liquid phase separation in model membranes. Elife 3 e01671
Huang B, Bates M and Zhuang X 2009 Super-resolution fluorescence microscopy. Annu. Rev. Biochem. 78 993–1016
Huang F, Hartwich TM, Rivera-Molina FE, Lin Y, Duim WC, Long JJ, Uchil PD, Myers JR, Baird MA, Mothes W et al. 2013 Video-rate nanoscopy using scmos camera-specific single-molecule localization algorithms. Nature Methods 10 653– 658
Jacobson K, Mouritsen OG and Anderson RG 2007 Lipid rafts: at a crossroad between cell biology and physics. Nat. Cell Biol. 9 7–14
Jans DC, Wurm CA, Riedel D, Wenzel D, Stagge F, Deckers M, Rehling P and Jakobs S 2013 Sted super-resolution microscopy reveals an array of Minos clusters along human mitochondria. Proc. Natl. Acad. Sci. USA 110 8936–8941
Kellner R, Baier C, Willig K, Hell S and Barrantes F 2007 Nanoscale organization of nicotinic acetylcholine receptors revealed by stimulated emission depletion microscopy. Neuroscience 144 135–143
Klar TA and Hell SW 1999 Subdiffraction resolution in far-field fluorescence microscopy. Opt. Lett. 24 954–956
Klar TA, Jakobs S, Dyba M, Egner A and Hell SW 2000 Fluorescence microscopy with diffraction resolution barrier broken by stimulated emission. Proc. Natl. Acad. Sci. U.S.A. 97 8206–8210
Koster S, Van Pee K, Hudel M, Leustik M, Rhinow D, Kuhlbrandt W, Chakraborty T and Yildiz O 2014 Crystal structure of listeriolysin o reveals molecular details of oligomerization and pore formation. Nat. Commun. 5 4690
Lau L, Lee YL, Sahl SJ, Stearns T and Moerner W 2012 Sted microscopy with optimized labeling density reveals 9-fold arrangement of a centriole protein. Biophys. J 102 2926–2935
Leung BO and Chou KC 2011 Review of super-resolution fluorescence microscopy for biology. Appl. Spectrosc. 65 967–980
Lingwood D and Simons K 2010 Lipid rafts as a membrane organizing principle. Science 327 46–50
Loschberger A, van de Linde S, Dabauvalle M-C, Rieger B, Heilemann M, Krohne G and Sauer M 2012 Super-resolution imaging visualizes the eightfold symmetry of gp210 proteins around the nuclear pore complex and resolves the central channel with nanometer resolution. J. Cell. Sci. 125 570–575
Manes S, del Real G and Martınez-A C 2003 Pathogens: raft hijackers. Nat. Rev. Immunol. 3 557–568
Meyer L, Wildanger D, Medda R, Punge A, Rizzoli SO, Donnert G and Hell SW 2008 Dual-color STED microscopy at 30-nm focal-plane resolution. Small. 4 1095–1100
Milo R and Phillips R 2015 Cell biology by the numbers. (Garland Science, Taylor & Francis Group)
Mitra K and Lippincott-Schwartz J 2010 Analysis of mitochondrial dynamics and functions using imaging approaches. Curr. Protoc. Cell Biol. 4 1–21
Mockl L, Lamb DC and Brauchle C 2014 Super-resolved fluorescence microscopy: Nobel prize in chemistry 2014 for Eric Betzig, Stefan Hell, and William E Moerner. Angew. Chem. Int. Ed. 53 13972– 13977
Moerner WE 2015 Single-molecule spectroscopy, imaging, and photocontrol: Foundations for super-resolution microscopy (Nobel lecture). Angew. Chem. Int. Ed. 54 8067–8093
Moon S, Yan R, Kenny SJ, Shyu Y, Xiang L, Li W and Xu K 2017 Spectrally resolved, functional super-resolution microscopy reveals nanoscale compositional heterogeneity in live-cell membranes. J. Am. Chem. Soc. 139 10944–10947
Mueller V, Ringemann C, Honigmann A, Schwarzmann G, Medda R, Leutenegger M, Polyakova S, Belov V, Hell S and Eggeling C 2011 Sted nanoscopy reveals molecular details of cholesterol-and cytoskeleton-modulated lipid interactions in living cells. Biophys. J. 101 1651–1660
Nabi IR and Le PU 2003 Caveolae/raft-dependent endocytosis. J. Cell Biol. 161 673–677
Nagerl UV, Willig KI, Hein B, Hell SW and Bonhoeffer T 2008 Live-cell imaging of dendritic spines by STED microscopy. Proc. Natl. Acad. Sci. USA 105 18982–18987
Orrit M 2014 Nobel Prize in chemistry: celebrating optical nanoscopy. Nat. Photonics 8 887
Owen DM and Gaus K 2013 Imaging lipid domains in cell membranes: the advent of super-resolution fluorescence microscopy. Front Plant Sci. 4 503
Owen DM, Rentero C, Rossy J, Magenau A, Williamson D, Rodriguez M and Gaus K 2010 Palm imaging and cluster analysis of protein heterogeneity at the cell surface. J. Biophotonics 3 446–454
Pelkmans L and Helenius A 2002 Endocytosis via caveolae. Traffic 3 311–320
Podobnik M, Marchioretto M, Zanetti M, Bavdek A, Kisovec M, Cajnko MM, Lunelli L, Dalla Serra M and Anderluh G 2015 Plasticity of listeriolysin o pores and its regulation by Ph and unique histidine. Sci. Rep. 5 9623
Roobala C and Basu JK 2017 Emergence of compositionally tunable nanoscale dynamical heterogeneity in model binary lipid biomembranes. Soft Matter 13 4598–4606
Rust MJ, Bates M and Zhuang X 2006 Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (storm). Nature Methods 3 793–796
Sahl SJ, Hell SW and Jakobs S 2017 Fluorescence nanoscopy in cell biology. Nat. Rev. Mol. Cell Biol. 18 685
Sarangi NK, Ayappa K and Basu JK 2017a Complex dynamics at the nanoscale in simple biomembranes. Sci Rep. 7 11173
Sarangi NK, Ayappa K, Visweswariah SS and Basu JK 2016a Nanoscale dynamics of phospholipids reveals an optimal assembly mechanism of pore-forming proteins in bilayer membranes. Phys. Chem. Chem. Phys. 18 29935–29945
Sarangi NK, Ayappa K, Visweswariah SS and Basu JK 2016b Super-resolution stimulated emission depletion-fluorescence correlation spectroscopy reveals nanoscale membrane reorganization induced by pore-forming proteins. Langmuir 32 9649–9657
Sarangi NK, Roobala C and Basu JK 2017b Unraveling complex nanoscale lipid dynamics in simple model biomembranes: Insights from fluorescence correlation spectroscopy in super-resolution stimulated emission depletion mode. Methods. https://doi.org/10.1016/j.ymeth.2017.11.011
Schermelleh L, Heintzmann R and Leonhardt H 2010 A guide to super-resolution fluorescence microscopy. J. Cell Biol. 190 165–175
Schmidt R, Wurm CA, Jakobs S, Engelhardt J, Egner A and Hell SW 2008 Spherical nanosized focal spot unravels the interior of cells. Nature Methods 5 539–544
Schmidt R, Wurm CA, Punge A, Egner A, Jakobs S and Hell SW 2009 Mitochondrial cristae revealed with focused light. Nano Lett. 9 2508–2510
Sengupta P, Jovanovic-Talisman T, Skoko D, Renz M, Veatch SL and Lippincott-Schwartz J 2011 Probing protein heterogeneity in the plasma membrane using palm and pair correlation analysis. Nature Methods 8 969–975
Sezgin E 2017 Super-resolution optical microscopy for studying membrane structure and dynamics. J. Phys. Condens. Matter 29 273001
Shim S-H, Xia C, Zhong G, Babcock HP, Vaughan JC, Huang B, Wang X, Xu C, Bi G-Q and Zhuang X 2012 Superresolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc. Natl. Acad. Sci. USA 109 13978–13983
Sieber JJ, Willig KI, Kutzner C, Gerding-Reimers C, Harke B, Donnert G, Rammner B, Eggeling C, Hell SW, Grubmuller H, et al. 2007 Anatomy and dynamics of a supramolecular membrane protein cluster. Science 317 1072–1076
Singer SJ and Nicolson GL 1972 The fluid mosaic model of the structure of cell membranes. Science 175 720–731
Stone MB, Shelby SA and Veatch SL 2017 Super-resolution microscopy: shedding light on the cellular plasma membrane. Chem. Rev. 117 7457–7477
Terasaki M 1995 Visualization of exocytosis during sea urchin egg fertilization using confocal microscopy. J. Cell Sci. 108 2293–2300
Vicidomini G, Hernandez IC, dAmora M, Zanacchi FC, Bianchini P and Diaspro A 2014 Gated cw-STED microscopy: a versatile tool for biological nanometer scale investigation. Methods 66 124–130
Wawrezinieck L, Rigneault H, Marguet D and Lenne P-F 2005 Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization. Biophys. J. 89 4029–4042
Wegner W, Ilgen P, Gregor C, van Dort J, Mott A C, Steffens Hv and Willig K I 2017 In vivo mouse and live cell STED microscopy of neuronal actin plasticity using far-red emitting fluorescent proteins. Sci. Rep. 7 11781
Wenger J, Conchonaud F, Dintinger J, Wawrezinieck L, Ebbesen TW, Rigneault H Marguet D and Lenne P-F 2007 Diffusion analysis within single nanometric apertures reveals the ultrafine cell membrane organization. Biophys. J. 92 913–919
Wildanger D, Rittweger E, Kastrup L and Hell SW 2008 Sted microscopy with a supercontinuum laser source. Opt. Express 16 9614–9621
Willig K, Harke B, Medda R and Hell SW 2007 Sted microscopy with continuous wave beams. Nature Methods 4 915–918
Willig KI, Rizzoli SO, Westphal V, Jahn R and Hell SW 2006 Sted microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature 440 935–939
Willig KI, Steffens H, Gregor C, Herholt A, Rossner MJ and Hell SW 2014 Nanoscopy of filamentous actin in cortical dendrites of a living mouse. Biophys. J. 106 L01–L03
Wilmes S, Staufenbiel M, Liße D, Richter CP, Beutel O, Busch KB, Hess ST and Piehler J 2012 Triple-color superresolution imaging of live cells: resolving submicroscopic receptor organization in the plasma membrane. Angew. Chem. Int. Ed. 51 4868–4871
Wu X, Toro L, Stefani E and Wu Y 2015a Ultrafast photon counting applied to resonant scanning STED microscopy. J. Microsc. 257 31–38
Wu Y, Wu X, Lu R, Zhang J, Toro L and Stefani E 2015b Resonant scanning with large field of view reduces photobleaching and enhances fluorescence yield in STED microscopy. Sci. Rep. 5
Xu K, Babcock H P and Zhuang X 2012 Dual-objective storm reveals three-dimensional filament organization in the actin cytoskeleton. Nature Methods 9 185–188
Acknowledgements
The authors thank DST for funding through a special DST – IRHPA project. I.I.P also thank the IISER Pune – Leica Micro Imaging Center at Indian Institute of Science Education and Research, Pune, for STED imaging. Authors are also grateful to Prof. K. Ganapathy Ayappa and Prof. Sandhya S. Visweswariah, IISc, for productive discussions. We are grateful to Dr. Nirod Kumar Sarangi for his help in some experimental work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roobala, C., Ilanila, I.P. & Basu, J.K. Applications of STED fluorescence nanoscopy in unravelling nanoscale structure and dynamics of biological systems. J Biosci 43, 471–484 (2018). https://doi.org/10.1007/s12038-018-9764-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-018-9764-3