ᅟ
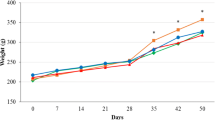
The main objective of this study was to investigate whether orally administered Korean grain larvae ethanol extract (GLE) had a bifidogenic effect in normal rats. Male Sprague–Dawley rats were divided into a negative control group (CO) and GLE orally administered (5.0, 7.0 and 9.0 mg/100 g body weight) groups. Thymus and spleen weights dose-dependently increased by 128.58% and 128.58%, respectively, but abdominal fat decreased by 19.18% after GLE administration compared with that in the CO group (p<0.05). Serum triglycerides, total cholesterol, low-density lipoprotein cholesterol, and glucose decreased by 30.26%, 7.33%, 27.20%, and 6.96%, respectively, whereas high-density lipoprotein cholesterol increased by 129.93% in the GLE groups compared with those in the CO group (p<0.05). IgG, IgM, IgA in the GLE groups increased 203.68%, 181.41%, and 238.25%, respectively, compared to that in the CO group (p<0.05). Bifidobacteria and Lactobacillus increased by 115.74% and 144.28%, whereas Bacteroides, Clostridium, Escherichia, and Streptococcus decreased by 17.37%, 17.46%, 21.25%, and 19.16%, respectively, in the GLE groups compared with those in the CO group (p<0.05). Total organic acids, acetic acid, and propionic acid increased by151.40%, 188.09%, and 150.17%, whereas butyric acid and valeric acid decreased by 40.65% and 49.24%, respectively, in the GLE groups as compared with those in the CO group (p < 0.05). These results suggest that Korean GLE improves the bifidogenic effect by increasing cecal organic acids and modulating gut microflora via a selective increase in Bifidobacterium in normal rats.
Similar content being viewed by others
References
Alejandra CC, Maria F, Nuria S, Cristina MV, Partrica RM and Clara RG 2009 Bifidogenic effect and stimulation of short chain fatty acid production in human faecal slurry cultures by oligosaccharides derived from lactose and lactulose. J. Dairy Res. 76 317–325
Bexfield A, Nigam Y, Thomas S and Ratcliffe NA 2004 Detection and partial characterisation of two antibacterial factors from the excretions/secretions of the medicinal maggot Luciliasericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA). Microbes Infect. 61 297–1304
Bienenstock J, Gauldie J and Perey DYE 1973 Synthesis of IgG, IgA, IgM by chicken tissues: immunofluorescent and 14C amino acid incorporation studies. J. Immunol. 111 1112–1118
Chunju A, Desen L and Rongqian D 2004 Analysis of antibacterial-relative proteins and peptides in housefly larvae. J. Hygiene Res. 33 86–88
Eckel RH, Grundy SM and Zimmer PZ 2005 The metabolic syndrome. Lancet. 365 1415–1428
Friedwald W, Levy R and Fredrickson D 1972 Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the prepararive ultracentrifuge. Clin. Chem. 18 499–502
Gibson GR and Wang X 1994 Bifidogenic properties of different types of fructooligosaccharides. Food Microbiol. 11 491–498
Higgins DA 1975 Physical and chemical properties of fowl immunoglobulins. Vet. Bull. 45 139–145
Jaklic D, Lapanje A, Zupanil K and Smrke D 2008 Selective antimicrobial activity of maggots against pathogenic bacteria. J. Med. Microbiol. 57 617–625
Liu X, Zeng A, Song T, Li L, Yang F, Wang Q, Wu B, Liu Y, et al. 2012 J. Biomater. Sci. 23 1107–1114
Lomax AR, Cheung LV, Tuohy KM, Noakes PS, Miles EA and Calder PC 2012 β2-1 Fructans have a bifidogenic effect in healthy middle-aged human subjects but do not alter immune responses examined in the absence of an in vivo immune challenge: results from a randomised controlled trial. Brit. J. Nutr. 108 1818–1828
Modler HW, Mckellar RC and Yaguchi M 1990 Bifidobacteria and bifidogenic factors. Can. Inst. Food Sci. Technol. J. 23 29–41
Napoli J, Brand-Miller J and Conway P 2003 Bifidogenic effects of feeding infant formula containing galacto-oligosaccharides in healthy formula-fed infants. Asia Pac. J. Clin. Nutr. 12 S60
National Institute of Health 1985 Public Health Service. Guide for the care and use of laboratory animals. NIH publication No. 86–23. Bethesda. NIH (1985)
Park BS 2008 Bifidogenic effects of inuloprebiotics in broiler chickens. J. Life Sci. 18 1693–1699
Park SO and Park BS 2012 Effects of grain larvae extracts on hepatotoxicity and blood lipid in obese rats. J. Anim. Vet. Adv. 11 988–994
Park BS, Jang A, Cho CR and Yoon KJ 2007 Seperation of antibaterial low molecular peptides from Muscadomesica maggot against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin resistant enterococcus (VRE); 2007 International Symposium and Annual Meeting. Kor. Soc. Food Sci. Nutr. October 17–19, 275
Park SO, Park BS and Oh JS 2010 Antibacterial activity of house fly maggot extracts against MRSA and VRE. J. Environ. Biol. 31 865–871
Patterson JA and Burkholder KM 2003 Application of prebiotics in poultry production. Poult. Sci. 82 627–631
Ratcliffe NA, Mello CB, Garcia ES, Butt TM and Azambuja P 2011 Insect natural products and processes: new treatments for human disease. Insect Biochem. Mol. Biol. 417 47–842
Reeves PG, Nielsen FH and Fahey GC 1993 AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123 1939–1951
Roberfroid MB 2000 Prebiotics and probiotics: are they functional foods. Am. J. Clin. Nutr. 71 1682S–1687S
Rolfe RD 2002 The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 130 396S–402S
Sánchez B, Urdaci MC and Margolles A 2010 Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa–bacteria interactions. Microbiology 156 3232–3242
Sánchez B, Ruiz L, Suárez A, de los Reyes-Gavilán CG and Margolles M 2011 Human caecum content modulates production of extracellular proteins by food and probiotic bacteria. FEMS Microbiol. Lett. 324 189–194
SAS 2005 SAS/STAT user's guide: statistics. Version 6.0 (Cary: SAS Institute Inc)
Sherman RA, Hall MJR and Thomas S 2000 Medicinal maggots: an ancient remedy for some contemporary afflictions. Annu. Rev. Entomol. 45 55–81
Veerman-Wauters G, Staelens S, Van de Broek H, Plaskie K, Wesling F, Roger LC, McCartney AL and Assam P 2011 Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J. Pediatr. Gastroenterol. Nutr. 52 763–771
Zhang WF, Li DF, Lu WQ and Yi GF 2003 Effects of isomalto-oligosacc-harides on broiler performance and intestinal microflora. Poult. Sci. 82 657–663
Acknowledgements
This study was supported by 2012 Kangwon National University (Project No.: C1008543-01-01), and 2014 Post-Doc Program Grant from Kangwon National University, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Yogesh Shouche
[Park S-O and Park B-S 2015 Bifidogenic effect of grain larvae extract on serum lipid, glucose and intestinal microflora in rats. J. Biosci.] DOI 10.1007/s12038-015-9540-6
Rights and permissions
About this article
Cite this article
Park, SO., Park, BS. Bifidogenic effect of grain larvae extract on serum lipid, glucose and intestinal microflora in rats. J Biosci 40, 513–520 (2015). https://doi.org/10.1007/s12038-015-9540-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-015-9540-6