ABSTRACT
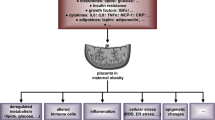
Mammals have three HP1 protein isotypes HP1β (CBX1), HP1γ (CBX3) and HP1α (CBX5) that are encoded by the corresponding genes Cbx1, Cbx3 and Cbx5. Recent work has shown that reduction of CBX3 protein in homozygotes for a hypomorphic allele (Cbx3 hypo) causes a severe postnatal mortality with around 99% of the homozygotes dying before weaning. It is not known what the causes of the postnatal mortality are. Here we show that Cbx3 hypo/hypo conceptuses are significantly reduced in size and the placentas exhibit a haplo-insufficiency. Late gestation Cbx3 hypo/hypo placentas have reduced mRNA transcripts for genes involved in growth regulation, amino acid and glucose transport. Blood vessels within the Cbx3 hypo/hypo placental labyrinth are narrower than wild-type. Newborn Cbx3 hypo/hypo pups are hypoglycemic, the livers are depleted of glycogen reserves and there is almost complete loss of stored lipid in brown adipose tissue (BAT). There is a 10-fold reduction in expression of the BAT-specific Ucp1 gene, whose product is responsible for non-shivering themogenesis. We suggest that it is the small size of the Cbx3 hypo/hypo neonates, a likely consequence of placental growth and transport defects, combined with a possible inability to thermoregulate that causes the severe postnatal mortality.




Similar content being viewed by others
References
Abe K, Naruse C, Kato T, Nishiuchi T, Saitou M and Asano M 2011 Loss of heterochromatin protein 1 gamma reduces the number of primordial germ cells via impaired cell cycle progression in mice. Biol. Reprod. 85 1013–1024
Alberts JR 1978 Huddling by rat pups: multisensory control of contact behavior. J. Comp. Physiol. Psychol. 92 220–230
Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, Roche D, Maison C, et al. 2012 An epigenetic silencing pathway controlling T helper 2 cell lineage, commitment. Nature 48 7249–253
Aucott R, Bullwinkel J, Yu Y, Shi W, Billur M, Brown JP, Menzel U, Kioussis D, et al. 2008 HP1-beta is required for development of the cerebral neocortex and neuromuscular junctions. J. Cell. Biol. 183 597–606
Billur M, Bartunik HD and Singh PB 2010 The essential function of HP1 beta: a case of the tail wagging the dog? Trends. Biochem. Sci. 35 115–123
Brown JP, Bullwinkel J, Baron-Luhr B, Billur M, Schneider P, Winking H and Singh PB 2010 HP1gamma function is required for male germ cell survival and spermatogenesis. Epigenetics Chromatin 3 9
Budge H, Mostyn A, Wilson V, Khong A, Walker AM, Symonds ME and Stephenson T 2002 The effect of maternal prolactin infusion during pregnancy on fetal adipose tissue development. J. Endocrinol. 174 427–433
Cannon B and Nedergaard J 2004 Brown adipose tissue: function and physiological significance. Physiol. Rev. 84 277–359
Canzio D, Larson A and Narlikar GJ 2014 Mechanisms of functional promiscuity byHP1proteins. Trends. Cell. Biol. 24 377–386
Chakravarty K, Cassuto H, Reshef L and Hanson RW 2005 Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit. Rev. Biochem. Mol. Biol. 40 129–154
Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M and Fowden AL 2010 Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J. Physiol. 588 527–538
Cogneville AM, Cividino N and Tordet-Caridroit C 1975 Lipid composition of brown adipose tissue as related to nutrition during the neonatal period in hypotrophic rats. J. Nutr. 105 982–988
Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, et al. 2002 Placental-specific IGF-II is a major modulator of placental and fetal, growth. Nature 417 945–948
Crandall DL, Hausman GJ and Kral JG 1997 A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic, perspectives. Microcirculation 4 211–232
Duttaroy AK 2009 Transport of fatty acids across the human placenta: a review. Prog. Lipid Res. 48(1) :52-61.
Eberlé D, Hegarty B, Bossard P, Ferré P and Foufelle F 2004 SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 86 839–848
Fedorenko A, Lishko PV and Kirichok Y 2012 Mechanism of fatty-acid-dependentUCP1uncoupling in brown fat mitochondria. Cell 151 400–413
Goldstein JL and Brown MS 1990 Regulation of the mevalonate pathway. Nature 343 425–430
Grewal SI and Elgin SC 2007 Transcription and RNA interference in the formation of heterochromatin. Nature 447 399–406
Haldane JBS 1926’On Being the Right Size’. Harper's Magazine.
Harshaw C and Alberts JR 2012 Group and individual regulation of physiology and behavior: a behavioral, thermographic, and acoustic study of mouse development. Physiol. Behav. 106 670–682
Hediger F and Gasser SM 2006 Heterochromatin protein 1: don't judge the book by its cover! Curr. Opin. Genet. Dev. 16 143–150
Horsley D, Hutchings A, Butcher GW and Singh PB 1996 M32, a murine homologue of Drosophila heterochromatin protein 1 (HP1), localises to euchromatin within interphase nuclei and is largely excluded from constitutive heterochromatin. Cytogenet. Cell. Genet. 73 308–311
Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS and Goldstein JL 2003 Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. U. S. A. 100 12027–12032
Jägerbauer EM, Fraser A, Herbst EW, Kothary R and Fundele R 1992 Parthenogenetic stem cells in postnatal mouse chimeras. Development 116 95–102
Jones DO, Cowell IG and Singh PB 2000 Mammalian chromodomain proteins: their role in genome organisation and expression. Bioessays 22 124–137
Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, et al. 2005 Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. U.S.A. 102 6207–6212
Kloosterboer HJ, Stoker-De Vries SA, Hulstaert CE and Hommes FA 1979 Quantitativeanalysis of morphological changes in skeletal muscle of the rat after hormone administration. Biol. Neonate. 35 106–112
Kurz H, Zechner U, Orth A and Fundele R 1999 Lack of correlation between placenta and offspring size in mouse interspecific crosses. Anat. Embryol. (Berl) 200 335–343
Lim S, Honek J, Xue Y, Seki T, Cao Z, Andersson P, Yang X, Hosaka K, et al. 2012 Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 7 606–615
Livak KJ and Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25 402–408
Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J and Cannon B 2000 Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J. Biol. Chem. 275 25073–25081
Nicholls DG and Locke RM 1984 Thermogenic mechanisms in brown fat. Physiol Rev. 64 1–64
Ohno H, Shinoda K, Ohyama K, Sharp LZ and Kajimura S 2013 EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 504 163–167
Puigserver P, Wu Z, Park CW, Graves R, Wright M and Spiegelman BM 1998 A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 92 829–839
Saunders WS, Chue C, Goebl M, Craig C, Clark RF, Powers JA, Eissenberg JC, Elgin SC, et al. 1993 Molecular cloning of a human homologue of Drosophila heterochromatin protein HP1 using anti-centromere autoantibodies with anti-chromo specificity. J. Cell. Sci. 104 573–582
Seale P, Bjyork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, et al. 2008 PRDM16 controls a brown fat/skeletal muscle switch. Nature 454 961–967
Sferruzzi-Perri AN, Vaughan OR, Coan PM, Suciu MC, Darbyshire R, Constancia M, Burton GJ and Fowden AL 2011 Placental-specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology 152 3202–3212
Singh PB 2010 What is the essential interaction of HP1? Genetika 46 1–6
Smallwood A, Hon GC, Jin F, Henry RE, Espinosa JM and Ren B 2012 CBX3 regulates efficient RNA processing genome-wide. Genome. Res. 22 1426–1436
Sokoloff G and Blumberg MS 2001 Competition and cooperation among huddling infant rats. Dev. Psychobiol. 39 65–75
Takada Y, Naruse C, Costa Y, Shirakawa T, Tachibana M, Sharif J, Kezuka-Shiotani F, et al. 2011 HP1gamma links histone methylation marks to meiotic synapsis in mice. Development 138 4207–4217
Turgeon B and Meloche S 2009 Interpreting neonatal lethal phenotypes in mouse mutants: insights into gene function and human diseases. Physiol. Rev. 8 91–26
Vakoc CR, Mandat SA, Olenchock BA and Blobel GA 2005 Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol. Cell. 19 381–391
Viengchareun S, Servel N, Feve B, Freemark M, Lombes M and Binart N 2008 Prolactin receptor signaling is essential for perinatal brown adipocyte function: a role for insulin-like growth factor-2. PLoS One 3 E1535
Watson ED and Cross JC 2005 Development of structures and transport in the mouse placenta. Physiology 20 180–193
Wiemers DO, L-j S, Ain R, Dai G and Soares MJ 2003 The mouse prolactin gene family, locus. Endochronolgy 144 313–325
Wood C, Kabat EA, Murphy LA and Goldstein IJ 1979 Immunochemical studies of the combining sites of the two isolectins, A4 and B4, isolated from Bandeiraea simplicifolia. Arch. Biochem. Biophys. 198 1–11
Wreggett KA, Hill F, James PS, Hutchings A, Butcher GW and Singh PB 1994 A mammalian homologue of Drosophila heterochromatin protein 1 (HP1) is a component of constitutive heterochromatin. Cytogenet. Cell. Genet. 66 99–103
Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, Yang Y, Opavska J, Wilson P, et al. 2003 Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421 942–947
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, et al. 1999 Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 98 115–124
Yu Y, Singh U, Shi W, Konno T, Soares MJ, Geyer R and Fundele R 2008 Influence of murine maternal diabetes on placental morphology, gene expression, and function. Arch. Physiol. Biochem. 114 99–110
Zechner U, Hemberger M, Constancia M, Orth A, Dragatsis I, Luttges A, Hameister H and Fundele R 2002 Proliferation and growth factor expression in abnormally enlarged placentas of mouse interspecific hybrids. Dev. Dyn. 224 125–134
Acknowledgements
We are grateful to Drs Nadine Binart, Miguel Constância and Mike Soares for critically reading the manuscript, and to Drs Irene and Kenneth Söderhäll for their help and support. This work was supported by the University of Uppsala, Department for Organismic Biology, and by the VR grant 621-2009-5715 from the Swedish Research Council to Kenneth Söderhäll.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Corresponding editor: Sanjeev Khosla
[Aydin E, Kloos D-P, Gay E, Jonker W, Hu L, Bullwinkel J, Brown JP, Manukyan M, Giera M, Singh PB and Fundele R 2015 A hypomorphic Cbx3 allele causes prenatal growth restriction and perinatal energy homeostasis defects. J. Biosci. 40 1–14] DOI 10.1007/s12038-015-9520-x
Rights and permissions
About this article
Cite this article
Aydin, E., Kloos, DP., Gay, E. et al. A hypomorphic Cbx3 allele causes prenatal growth restriction and perinatal energy homeostasis defects. J Biosci 40, 325–338 (2015). https://doi.org/10.1007/s12038-015-9520-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-015-9520-x