Abstract
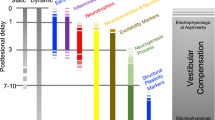
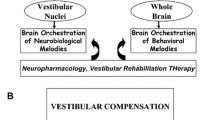
Vestibular compensation is a physiological response of the vestibular organs within the inner ear. This adaptation manifests during consistent exposure to acceleration or deceleration, with the vestibular organs incrementally adjusting to such changes. The molecular underpinnings of vestibular compensation remain to be fully elucidated, yet emerging studies implicate associations with neuroplasticity and signal transduction pathways. Throughout the compensation process, the vestibular sensory neurons maintain signal transmission to the central equilibrium system, facilitating adaptability through alterations in synaptic transmission and neuronal excitability. Notable molecular candidates implicated in this process include variations in ion channels and neurotransmitter profiles, as well as neuronal and synaptic plasticity, metabolic processes, and electrophysiological modifications. This study consolidates the current understanding of the molecular events in vestibular compensation, augments the existing research landscape, and evaluates contemporary therapeutic strategies. Furthermore, this review posits potential avenues for future research that could enhance our comprehension of vestibular compensation mechanisms.


Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- ACh:
-
Acetylcholine
- AchE:
-
Acetylcholinesterase
- ACTH:
-
Adrenocorticotropic hormone
- AraC:
-
Cytosine-β-D arabinofuranoside
- AVP:
-
Arginine vasopressin
- BDNF:
-
Brain-derived neurotrophic factor
- BK:
-
Calcium-activated K+ Big conductance
- BrdU:
-
Bromodeoxyuridine
- CAMKII:
-
Calmodulin-dependent kinase II
- CNS,:
-
Central nervous system
- CO:
-
Cytochrome oxygenase
- CRH,:
-
Corticotropin-releasing hormone
- ENS:
-
Enteric nervous system
- HCN:
-
Hyperpolarization-activated cyclic nucleotide-gated
- HPA:
-
Hypothalamus-pituitary-adrenal
- IEGs:
-
Immediate early genes
- IO:
-
Inferior olive
- KCC2:
-
K(+)-Cl(-) cotransporter isoform 2
- L-T4:
-
L-Thyroxine
- LVN:
-
Lateral vestibular nucleus
- MnSOD:
-
Manganese superoxide dismutase
- MVN:
-
Medial vestibular nucleus
- NF-κB:
-
Nuclear factor kappa B
- NGF:
-
Nerve growth factor
- NPH:
-
Nucleus prepositus hypoglossi
- NT:
-
Nerve growth factor
- NT3:
-
Neurotrophic factor 3
- NT4:
-
Neurotrophic factor 4
- NT5:
-
Neurotrophic factor 5
- NTs:
-
Neurotrophins
- PKC:
-
Protein kinase C
- PNS:
-
Peripheral nervous system
- PVN:
-
Paraventricular nucleus
- SK:
-
Calcium-activated K+ small conductance
- TH:
-
Thyroid hormone
- TNF-α:
-
Tumor necrosis factor alpha
- TrkB:
-
Tyrosine receptor kinase B
- UBCs:
-
Unipolar brush cells
- UL:
-
Unilateral labyrinthectomy
- UVD:
-
Unilateral vestibular deafferentation
- UVL:
-
Unilateral vestibular lesion
- UVN:
-
Unilateral vestibular neurectomy
- VN:
-
Vestibular nucleus
- VNCs:
-
Vestibular nucleus complexes
- VOR:
-
Vestibular-ocular reflex
- 2DG:
-
2-deoxyglucose
References
Ferrè ER, Longo MR, Fiori F et al (2013) Vestibular modulation of spatial perception. Front Hum Neurosci 7:660
Angelaki DE, Klier EM, Snyder LH (2009) A vestibular sensation: probabilistic approaches to spatial perception. Neuron 64:448–461
Lacour M, Borel L (1993) Vestibular control of posture and gait. Arch Ital Biol 131:81–104
Lacour M, Tighilet B (2010) Plastic events in the vestibular nuclei during vestibular compensation: the brain orchestration of a “deafferentation” code. Restor Neurol Neurosci 28:19–35
Ris L, de Waele C, Serafin M et al (1995) Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J Neurophysiol 74:2087–2099
Smith PF, Curthoys IS (1989) Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev 14:155–180
Curthoys IS (2000) Vestibular compensation and substitution. Curr Opin Neurol 13:27–30
Straka H, Vibert N, Vidal PP et al (2005) Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76:349–392
Dugas JC, Cuellar TL, Scholze A et al (2010) Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 65:597–611
Beraneck M, Idoux E (2012) Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front Neurol 3:25
Kaufman GD, Anderson JH, Beitz AJ (1992) Brainstem Fos expression following acute unilateral labyrinthectomy in the rat. Neuroreport 3:829–832
Kitahara T, Takeda N, Saika T et al (1995) Effects of MK801 on Fos expression in the rat brainstem after unilateral labyrinthectomy. Brain Res 700:182–190
Cirelli C, Pompeiano M, D’Ascanio P et al (1996) c-fos Expression in the rat brain after unilateral labyrinthectomy and its relation to the uncompensated and compensated stages. Neuroscience 70:515–546
Darlington CL, Lawlor P, Smith PF et al (1996) Temporal relationship between the expression of fos, jun and krox-24 in the guinea pig vestibular nuclei during the development of vestibular compensation for unilateral vestibular deafferentation. Brain Res 735:173–176
Gustave Dit Duflo S, Gestreau C, Tighilet B et al (1999) Fos expression in the cat brainstem after unilateral vestibular neurectomy. Brain Res 824:1–17
Kitahara T, Fukushima M, Takeda N et al (2000) Effects of pre-flocculectomy on Fos expression and NMDA receptor-mediated neural circuits in the central vestibular system after unilateral labyrinthectomy. Acta Otolaryngol 120:866–871
Kim MS, Kim JH, Jin Y-Z et al (2002) Temporal changes of cFos-like protein expression in medial vestibular nuclei following arsanilate-induced unilateral labyrinthectomy in rats. Neurosci Lett 319:9–12
El Mahmoudi N, Marouane E, Rastoldo G et al (2022) Microglial dynamics modulate vestibular compensation in a rodent model of vestibulopathy and condition the expression of plasticity mechanisms in the deafferented vestibular nuclei. Cells 11:2693
El Mahmoudi N, Rastoldo G, Marouane E et al (2021) Breaking a dogma: acute anti-inflammatory treatment alters both post-lesional functional recovery and endogenous adaptive plasticity mechanisms in a rodent model of acute peripheral vestibulopathy. J Neuroinflammation 18:183
Baizer JS, Corwin WL, Baker JF (2010) Otolith stimulation induces c-Fos expression in vestibular and precerebellar nuclei in cats and squirrel monkeys. Brain Res 1351:64–73
Liberge M, Manrique C, Bernard-Demanze L et al (2010) Changes in TNFα, NFκB and MnSOD protein in the vestibular nuclei after unilateral vestibular deafferentation. J Neuroinflammation 7:91
Bolger C, Sansom AJ, Smith PF et al (1999) An antisense oligonucleotide to brain-derived neurotrophic factor delays postural compensation following unilateral labyrinthectomy in guinea pig. Neuroreport 10:1485–1488
Montcouquiol ME, Sans NA, Travo C et al (2000) Detection and localization of BDNF in vestibular nuclei during the postnatal development of the rat. Neuroreport 11:1401–1405
Maingay MG, Sansom AJ, Kerr DR et al (2000) The effects of intra-vestibular nucleus administration of brain-derived neurotrophic factor (BDNF) on recovery from peripheral vestibular damage in guinea pig. Neuroreport 11:2429–2432
Dutheil S, Watabe I, Sadlaoud K et al (2016) BDNF signaling promotes vestibular compensation by increasing neurogenesis and remodeling the expression of potassium-chloride cotransporter KCC2 and GABAA receptor in the vestibular nuclei. J Neurosci 36:6199–6212
Mao D, He Z, Xuan W et al (2021) Effect and mechanism of BDNF/TrkB signaling on vestibular compensation. Bioengineered 12:11823–11836
Dutheil S, Escoffier G, Gharbi A et al (2013) GABA(A) receptor agonist and antagonist alter vestibular compensation and different steps of reactive neurogenesis in deafferented vestibular nuclei of adult cats. J Neurosci 33:15555–15566
Yamanaka T, Him A, Cameron SA et al (2000) Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol 523(Pt 2):413–424
Gliddon CM, Darlington CL, Smith PF (2005) GABAergic systems in the vestibular nucleus and their contribution to vestibular compensation. Prog Neurobiol 75:53–81
Heskin-Sweezie R, Titley HK, Baizer JS et al (2010) Type B GABA receptors contribute to the restoration of balance during vestibular compensation in mice. Neuroscience 169:302–314
Shao M, Reddaway R, Hirsch JC et al (2012) Presynaptic GABA(B) receptors decrease neurotransmitter release in vestibular nuclei neurons during vestibular compensation. Neuroscience 223:333–354
Phelan KD, Gallagher JP (1992) Direct muscarinic and nicotinic receptor-mediated excitation of rat medial vestibular nucleus neurons in vitro. Synapse 10:349–358
Sun Y, Waller HJ, Godfrey DA et al (2002) Spontaneous activity in rat vestibular nuclei in brain slices and effects of acetylcholine agonists and antagonists. Brain Res 934:58–68
Tighilet B, Trottier S, Mourre C et al (2006) Changes in the histaminergic system during vestibular compensation in the cat. J Physiol 573:723–739
Tighilet B, Mourre C, Trottier S et al (2007) Histaminergic ligands improve vestibular compensation in the cat: behavioural, neurochemical and molecular evidence. Eur J Pharmacol 568:149–163
Tighilet B, Mourre C, Lacour M (2014) Plasticity of the histamine H3 receptors after acute vestibular lesion in the adult cat. Front Integr Neurosci 7:87
Tighilet B, Lacour M (2001) Gamma amino butyric acid (GABA) immunoreactivity in the vestibular nuclei of normal and unilateral vestibular neurectomized cats. Eur J Neurosci 13:2255–2267
Tighilet B, Brezun JM, Sylvie GDD et al (2007) New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur J Neurosci 25:47–58
Bergquist F, Ruthven A, Ludwig M et al (2006) Histaminergic and glycinergic modulation of GABA release in the vestibular nuclei of normal and labyrinthectomised rats. J Physiol 577:857–868
Chen Z-P, Zhang X-Y, Peng S-Y et al (2019) Histamine H1 receptor contributes to vestibular compensation. J Neurosci 39:420–433
Alice C, Paul AE, Sansom AJ et al (1998) The effects of steroids on vestibular compensation and vestibular nucleus neuronal activity in the guinea pig. J Vestib Res 8:201–207
Yamamoto T, Yamanaka T, Matsunaga T (2000) The effect of stress application on vestibular compensation. Acta Otolaryngol 120:504–507
Licata F, Li Volsi G, Maugeri G et al (1993) Effects of noradrenaline on the firing rate of vestibular neurons. Neuroscience 53:149–158
Podda MV, Johnston AR, Tolu E et al (2001) Modulation of rat medial vestibular nucleus neurone activity by vasopressin and noradrenaline in vitro. Neurosci Lett 298:91–94
Di Mauro M, Bronzi D, Li Volsi G et al (2008) Noradrenaline modulates neuronal responses to GABA in vestibular nuclei. Neuroscience 153:1320–1331
Cai J, Li J, Mao Y et al (2013) Immunohistochemical localization of α2-adrenergic receptors in the neonatal rat cochlea and the vestibular labyrinth. J Mol Neurosci 51:1010–1020
Wersinger E, Gaboyard-Niay S, Travo C et al (2013) Symptomatic treatment of vestibular deficits: therapeutic potential of histamine H4 receptors. J Vestib Res 23:153–159
Sadlaoud K, Tazerart S, Brocard C et al (2010) Differential plasticity of the GABAergic and glycinergic synaptic transmission to rat lumbar motoneurons after spinal cord injury. J Neurosci 30:3358–3369
Nelson AB, Faulstich M, Moghadam S et al (2017) BK channels are required for multisensory plasticity in the oculomotor system. Neuron 93:211–220
van Welie I, du Lac S (2011) Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. J Neurophysiol 105:1651–1659
Tighilet B, Bourdet A, Péricat D et al (2021) SK channels modulation accelerates equilibrium recovery in unilateral vestibular neurectomized rats. Pharmaceuticals:14. https://doi.org/10.3390/ph14121226
Tighilet B, Leonard J, Mourre C et al (2019) Apamin treatment accelerates equilibrium recovery and gaze stabilization in unilateral vestibular neurectomized cats: cellular and behavioral aspects. Neuropharmacology. 144:133–142
Tighilet B, Chabbert C (2019) Adult neurogenesis promotes balance recovery after vestibular loss. Prog Neurobiol 174:28–35
Campos-Torres A, Touret M, Vidal PP et al (2005) The differential response of astrocytes within the vestibular and cochlear nuclei following unilateral labyrinthectomy or vestibular afferent activity blockade by transtympanic tetrodotoxin injection in the rat. Neuroscience 130:853–865
Dutheil S, Lacour M, Tighilet B (2011) Neurogenic potential of the vestibular nuclei and behavioural recovery time course in the adult cat are governed by the nature of the vestibular damage. PLoS One 6:e22262
Dutheil S, Brezun JM, Leonard J et al (2009) Neurogenesis and astrogenesis contribution to recovery of vestibular functions in the adult cat following unilateral vestibular neurectomy: cellular and behavioral evidence. Neuroscience 164:1444–1456
Rastoldo G, El Mahmoudi N, Marouane E et al (2021) Adult and endemic neurogenesis in the vestibular nuclei after unilateral vestibular neurectomy. Prog Neurobiol 196:101899
Raymond J, Ez-Zaher L, Demêmes D et al (1991) Quantification of synaptic density changes in the medial vestibular nucleus of the cat following vestibular neurectomy. Restor Neurol Neurosci 3:197–203
Li H, Dokas LA, Godfrey DA et al (2002) Remodeling of synaptic connections in the deafferented vestibular nuclear complex. J Vestib Res 12:167–183
C T, S G-N, C C. (2012) Plasticity of Scarpa’s ganglion neurons as a possible basis for functional restoration within vestibular endorgans. Front Neurol:3. https://doi.org/10.3389/fneur.2012.00091
Curran T, Abate C, Cohen DR et al (1990) Inducible proto-oncogene transcription factors: third messengers in the brain? Cold Spring Harb Symp Quant Biol 55:225–234
Klein RL, Muir D, King MA et al (1999) Long-term actions of vector-derived nerve growth factor or brain-derived neurotrophic factor on choline acetyltransferase and Trk receptor levels in the adult rat basal forebrain. Neuroscience 90:815–821
Zhang J, Zhang D, McQuade JS et al (2002) c-fos regulates neuronal excitability and survival. Nat Genet 30:416–420
Robertson HA (1992) Immediate-early genes, neuronal plasticity, and memory. Biochem Cell Biol 70:729–737
Ito T, Tatsumi K, Takimoto Y et al (2019) Vestibular compensation after vestibular dysfunction induced by arsanilic acid in mice. Brain Sci 9:329
Fukuda J, Matsuda K, Sato G et al (2021) Effects of betahistine on the development of vestibular compensation after unilateral labyrinthectomy in rats. Brain Sci 11:360
von Bartheld CS, Fritzsch B (2006) Comparative analysis of neurotrophin receptors and ligands in vertebrate neurons: tools for evolutionary stability or changes in neural circuits? Brain Behav Evol 68:157–172
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309
Guo W, Nagappan G, Lu B (2018) Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev Neurobiol 78:647–659
Johnstone A, Mobley W (2020) Local TrkB signaling: themes in development and neural plasticity. Cell Tissue Res 382:101–111
Benítez-Temiño B, Morcuende S, Mentis GZ et al (2004) Expression of Trk receptors in the oculomotor system of the adult cat. J Comp Neurol 473:538–552
Zhang FX, Lai CH, Lai SK et al (2003) Neurotrophin receptor immunostaining in the vestibular nuclei of rats. Neuroreport 14:851–855
Zhou L, Zhou W, Zhang S et al (2015) BDNF signaling in the rat cerebello-vestibular pathway during vestibular compensation: BDNF signaling in vestibular compensation. FEBS J 282:3579–3591
Ernfors P, Van De Water T, Loring J, Jaenisch R et al (1995) Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron 14:1153–1164. https://doi.org/10.1016/0896-6273(95)90263-5
Johnston AR, Him A, Dutia MB (2001) Differential regulation of GABA(A) and GABA(B) receptors during vestibular compensation. Neuroreport 12:597–600
Bergquist F, Ludwig M, Dutia MB (2008) Role of the commissural inhibitory system in vestibular compensation in the rat. J Physiol 586:4441–4452
Cm G, Cl D, Pf S (2005) Effects of chronic infusion of a GABAA receptor agonist or antagonist into the vestibular nuclear complex on vestibular compensation in the guinea pig. J Pharmacol Exp Ther 313:1126–1135. https://doi.org/10.1124/jpet.104.082172
de Waele C, Mühlethaler M, Vidal PP (1995) Neurochemistry of the central vestibular pathways. Brain Res Brain Res Rev 20:24–46
Torte-Hoba MP, Courjon JH, Leroy MH et al (1996) Acetylcholinesterase activity changes in medial vestibular complex after hemilabyrinthectomy in the rat. J Vestib Res 6:61–70
Monzani D, Genovese E, Marrara A et al (2010) Stimulation of the cholinergic neurotransmissions enhances the efficacy of vestibular rehabilitation. Acta Otorhinolaryngol Ital 30:11–19
Herman JP, Figueiredo H, Mueller NK et al (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180
Cm G, Cl D, Pf S (2003) Activation of the hypothalamic-pituitary-adrenal axis following vestibular deafferentation in pigmented guinea pig. Brain Res 964:306–310. https://doi.org/10.1016/s0006-8993(02)04086-6
Tighilet B, Manrique C, Lacour M (2009) Stress axis plasticity during vestibular compensation in the adult cat. Neuroscience 160:716–730
Johnston AR, Seckl JR, Dutia MB (2002) Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J Physiol 545:903–911
Zhang X-Y, Yu L, Zhuang Q-X et al (2013) Postsynaptic mechanisms underlying the excitatory action of histamine on medial vestibular nucleus neurons in rats. Br J Pharmacol 170:156–169
B T, S T, M L. (2005) Dose- and duration-dependent effects of betahistine dihydrochloride treatment on histamine turnover in the cat. Eur J Pharmacol:523. https://doi.org/10.1016/j.ejphar.2005.09.017
Zhuang Q-X, Wu Y-H, Wu G-Y et al (2013) Histamine excites rat superior vestibular nuclear neurons via postsynaptic H1 and H2 receptors in vitro. Neurosignals. 21:174–183
Horii A, Takeda N, Matsunaga T et al (1993) Effect of unilateral vestibular stimulation on histamine release from the hypothalamus of rats in vivo. J Neurophysiol 70:1822–1826
Yabe T, de Waele C, Serafin M et al (1993) Medial vestibular nucleus in the guinea-pig: histaminergic receptors. II. An in vivo study. Exp Brain Res 93:249–258
Li B, Zhang X-Y, Yang A-H et al (2016) Histamine increases neuronal excitability and sensitivity of the lateral vestibular nucleus and promotes motor behaviors via HCN channel coupled to H2 receptor. Front Cell Neurosci 10:300
Kokaia M (2011) Seizure-induced neurogenesis in the adult brain. Eur J Neurosci 33:1133–1138
Coull JAM, Beggs S, Boudreau D et al (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438:1017–1021
Li X, Tayoun AA, Song Z et al (2018) Ca2+-activated K+ channels reduce network excitability, improving adaptability and energetics for transmitting and perceiving sensory information. J Neurosci 39:7132–7154
Tighilet B, Bourdet A, Péricat D et al (2021) SK channels modulation accelerates equilibrium recovery in unilateral vestibular neurectomized rats. Pharmaceuticals (Basel) 14:1226
Gonzalez-Perez O, Quiñones-Hinojosa A (2012) Astrocytes as neural stem cells in the adult brain. J Stem Cells 7:181–188
Chung W-S, Clarke LE, Wang GX et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 504:394–400
Chung W-S, Allen NJ, Eroglu C (2015) Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7:a020370
Lacour M, Dutheil S, Tighilet B et al (2009) Tell me your vestibular deficit, and I’ll tell you how you’ll compensate. Ann N Y Acad Sci 1164:268–278
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: friend or foe? Mol Neurobiol 54:8071–8089
Russo MV, McGavern DB (2016) Inflammatory neuroprotection following traumatic brain injury. Science. 353:783–785
Jurga AM, Paleczna M, Kuter KZ (2020) Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci 14:198
Tanaka T, Murakami K, Bando Y et al (2017) Microglia support ATF3-positive neurons following hypoglossal nerve axotomy. Neurochem Int 108:332–342
Clark AK, Gruber-Schoffnegger D, Drdla-Schutting R et al (2015) Selective activation of microglia facilitates synaptic strength. J Neurosci 35:4552–4570
Sienko KH, Whitney SL, Carender WJ et al (2017) The role of sensory augmentation for people with vestibular deficits: real-time balance aid and/or rehabilitation device? J Vestib Res 27:63–76
Chaisuksunt V, Zhang Y, Anderson PN et al (2000) Axonal regeneration from CNS neurons in the cerebellum and brainstem of adult rats: correlation with the patterns of expression and distribution of messenger RNAs for L1, CHL1, c-jun and growth-associated protein-43. Neuroscience 100:87–108
Dyhrfjeld-Johnsen J, Gaboyard-Niay S, Broussy A et al (2013) Ondansetron reduces lasting vestibular deficits in a model of severe peripheral excitotoxic injury. J Vestib Res 23:177–186
Gaboyard-Niay S, Travo C, Saleur A et al (2016) Correlation between afferent rearrangements and behavioral deficits after local excitotoxic insult in the mammalian vestibule: a rat model of vertigo symptoms. Dis Model Mech 9:1181–1192
Genovese T, Impellizzeri D, Ahmad A et al (2013) Post-ischaemic thyroid hormone treatment in a rat model of acute stroke. Brain Res 1513:92–102
Shulga A, Blaesse A, Kysenius K et al (2009) Thyroxin regulates BDNF expression to promote survival of injured neurons. Mol Cell Neurosci 42:408–418
Liu Y-Y, Brent GA (2021) The role of thyroid hormone in neuronal protection. Compr Physiol 11:2075–2095
Talhada D, Santos CRA, Gonçalves I et al (2019) Thyroid hormones in the brain and their impact in recovery mechanisms after stroke. Front Neurol 10:1103
Rastoldo G, Marouane E, El-Mahmoudi N et al (2022) L-thyroxine improves vestibular compensation in a rat model of acute peripheral vestibulopathy: cellular and behavioral aspects. Cells 11:684
Bringuier CM, Hatat B, Boularand R et al (2022) Characterization of thyroid hormones antivertigo effects in a rat model of excitotoxically-induced vestibulopathy. Front Neurol 13:877319
Wong-Riley MT (1989) Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci 12:94–101
Tighilet B, Leonard J, Bernard-Demanze L et al (2015) Comparative analysis of pharmacological treatments with N-acetyl-DL-leucine (Tanganil) and its two isomers (N-acetyl-L-leucine and N-acetyl-D-leucine) on vestibular compensation: behavioral investigation in the cat. Eur J Pharmacol 769:342–349
Günther L, Beck R, Xiong G et al (2015) N-acetyl-L-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus. PLoS One 10:e0120891
Vibert N, Vidal PP (2001) In vitro effects of acetyl-DL-leucine (tanganil) on central vestibular neurons and vestibulo-ocular networks of the guinea-pig. Eur J Neurosci 13:735–748
Marouane E, El Mahmoudi N, Rastoldo G et al (2021) Sensorimotor rehabilitation promotes vestibular compensation in a rodent model of acute peripheral vestibulopathy by promoting microgliogenesis in the deafferented vestibular nuclei. Cells 10:3377
Santos-Pata D, Bellmunt A, Mozo RMSS et al (2022) Impaired reaching adaptation links to vestibular symptoms. medRxiv. https://doi.org/10.1101/2022.02.07.22270496
Beraneck M, Mckee JL, Aleisa M et al (2008) Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol 100(2):945–958
Menzies JRW, Porrill J, Dutia M et al (2010) Synaptic plasticity in medial vestibular nucleus neurons: comparison with computational requirements of VOR adaptation. PLoS One 5:e13182
McCabe BF, Ryu JH (1969) Experiments on vestibular compensation. Laryngoscope 79:1728–1736
Smith PF (2020) Why the cerebellar shutdown/clampdown hypothesis of vestibular compensation is inconsistent with neurophysiological evidence. J Vestib Res 30:295–303
Liu D, Wang J, Zhou L et al (2023) Differential modulation of cerebellar flocculus unipolar brush cells during vestibular compensation. Biomedicines 11:1298
Liu D, Wang J, Tian E et al (2024) mGluR1/IP3/ERK signaling pathway regulates vestibular compensation in ON UBCs of the cerebellar flocculus. CNS Neurosci Ther 30:e14419
Lacour M (2006) Restoration of vestibular function: basic aspects and practical advances for rehabilitation. Curr Med Res Opin 22:1651–1659
Newlands SD, Dara S, Kaufman GD (2005) Relationship of static and dynamic mechanisms in vestibuloocular reflex compensation. Laryngoscope 115:191–204
Rogge A-K, Röder B, Zech A et al (2018) Exercise-induced neuroplasticity: balance training increases cortical thickness in visual and vestibular cortical regions. Neuroimage 179:471–479
Facchini J, Rastoldo G, Xerri C et al (2021) Unilateral vestibular neurectomy induces a remodeling of somatosensory cortical maps. Prog Neurobiol 205:102119
Raiser TM, Flanagin VL, Duering M et al (2020) The human corticocortical vestibular network. Neuroimage 223:117362
Li F, Feng Y, Liu H et al (2022) Gut microbiome and metabolome changes in mice with acute vestibular deficit. Front Cell Infect Microbiol 12:821780
Erny D, Hrabě de Angelis AL, Jaitin D et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18:965–977
Matcovitch-Natan O, Winter DR, Giladi A et al (2016) Microglia development follows a stepwise program to regulate brain homeostasis. Science 353:aad8670
Han RT, Kim RD, Molofsky AV et al (2021) Astrocyte-immune cell interactions in physiology and pathology. Immunity 54:211–224
Kingsley TR, Nekvasil NP, Snyder DL (1991) The influence of dietary restriction, germ-free status, and aging on adrenal catecholamines in Lobund-Wistar rats. J Gerontol 46:B135–B141
Smith DK, Kassam T, Singh B et al (1992) Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J Bacteriol 174:5820–5826
O’Mahony SM, Clarke G, Borre YE et al (2015) Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277:32–48
Hata T, Asano Y, Yoshihara K et al (2017) Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS One 12:e0180745
Strandwitz P, Kim KH, Terekhova D et al (2019) GABA-modulating bacteria of the human gut microbiota. Nat Microbiol 4:396–403
Chen H-L, Tan C-T, Wu C-C et al (2022) Effects of diet and lifestyle on audio-vestibular dysfunction in the elderly: a literature review. Nutrients 14:4720
Funding
This study was supported by grants from National Natural Science Foundation of China (82271410), Guangzhou Science and Technology Program key projects (202206010097)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Junyu Wu, Xue Xu, Shifeng Zhang, Minping Li, Yuemin Qiu, Gengxin Lu, and Zhihui Zheng. The first draft of the manuscript was written by Junyu Wu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, J., Xu, X., Zhang, S. et al. Plastic Events of the Vestibular Nucleus: the Initiation of Central Vestibular Compensation. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04208-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04208-2