Abstract
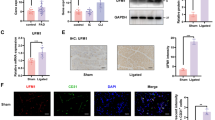
Stroke remains the 3rd leading cause of long-term disability globally. Over the past decade, mesenchymal stem cell (MSC) transplantation has been proven as an effective therapy for ischemic stroke. However, the mechanism of MSC-derived exosomal lncRNAs during cerebral ischemia/reperfusion (I/R) remains ambiguous. The oxygen–glucose deprivation/reoxygenation (OGD/R) and middle cerebral artery occlusion (MCAO) rat model were generated. MSCs were isolated and characterized by flow cytometry and histochemical staining, and MSC exosomes were purified and characterized by transmission electron microscopy, flow cytometry and Western blot. Western blot, RT-qPCR and ELISA assay were employed to examine the expression or secretion of key molecules. CCK-8 and TUNEL assays were used to assess cell viability and apoptosis. RNA immunoprecipitation and RNA pull-down were used to investigate the direct association between krüppel-like factor 3 antisense RNA 1 (KLF3-AS1) and musashi-1(MSI1). Yin Yang 1 (YY1)-mediated transcriptional regulation was assessed by chromatin immunoprecipitation and luciferase assays. The histological changes and immunoreactivity of key molecules in brain tissues were examined by H&E and immunohistochemistry. MSCs were successfully isolated and exhibited directionally differential potentials. MSC exosomal KLF3-AS1 alleviated OGD/R-induced inflammation in SK-N-SH and SH-SY5Y cells via modulating Sphk1. Mechanistical studies showed that MSI1 positively regulated KLF3-AS1 expression through its direct binding to KLF3-AS1. YY1 was identified as a transcription activator of MSI1 in MSCs. Functionally, YY1/MSI1 axis regulated the release of MSC exosomal KLF3-AS1 to modulate sphingosine kinase 1 (Sphk1)/NF-κB pathway, thereby ameliorating OGD/R- or cerebral I/R-induced injury. MSCs promote the release of exosomal KLF3-AS1 to regulate Sphk1 through YY1/MSI axis and improve cerebral I/R injury.









Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ATCC:
-
American type culture collection
- BBB:
-
Blood-brain barrier
- BS:
-
Binding site
- CCA:
-
Common carotid artery
- ceRNA:
-
Competing endogenous
- ChIP:
-
Chromatin immunoprecipitation
- CM:
-
Conditioned media
- CNS:
-
Central nervous system
- H&E:
-
Hematoxylin and eosin
- I/R:
-
Ischemia/reperfusion
- KLF3-AS1:
-
Krüppel-like factor 3 antisense RNA 1
- lncRNA:
-
Long non-coding RNA
- MCAO:
-
Middle cerebral artery occlusion
- MI:
-
Myocardial infarction
- MSC:
-
Mesenchymal stem cell
- MSI1:
-
Musashi-1
- ncRNA:
-
Non-coding RNA
- NF-κB:
-
Nuclear factor-kappa B
- NO:
-
Nitric oxide
- OGD/R:
-
Oxygen-glucose deprivation/reoxygenation
- RBP:
-
RNA-binding protein
- RIP:
-
RNA immunoprecipitation
- ROS:
-
Reactive oxygen species
- SAH:
-
Subarachnoid hemorrhage
- S1P:
-
Sphingosine 1-phosphate
- Sphk1:
-
Sphingosine kinase 1
- TEM:
-
Transmission electron microscopy
- TRAF2:
-
Tumor necrosis factor receptor-associated factor 2
- Tregs:
-
Regulatory T cells
- TTC:
-
2,3,5-tripthenyltetrazolum chloride
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick and labeling
- YY1:
-
Yin Yang 1
References
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE et al (2022) Heart disease and stroke statistics-2022 update: a report from the american heart association. Circulation 145(8):e153–e639. https://doi.org/10.1161/CIR.0000000000001052
Wang YC, Kapellusch J, Garg A (2014) Important factors influencing the return to work after stroke. Work 47(4):553–559. https://doi.org/10.3233/WOR-131627
Phipps MS, Cronin CA (2020) Management of acute ischemic stroke. BMJ 368:l6983. https://doi.org/10.1136/bmj.l6983
Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C (2018) Reperfusion therapy in acute ischemic stroke: dawn of a new era? BMC Neurol 18(1):8. https://doi.org/10.1186/s12883-017-1007-y
Imran R, Mohamed GA, Nahab F (2021) Acute reperfusion therapies for acute ischemic stroke. J Clin Med 10(16). https://doi.org/10.3390/jcm10163677
Nour M, Scalzo F, Liebeskind DS (2013) Ischemia-reperfusion injury in stroke. Interv Neurol 1(3–4):185–199. https://doi.org/10.1159/000353125
Mandalaneni K, Rayi A, Jillella DV (2022) Stroke reperfusion injury. In: StatPearls. Treasure Island (FL). https://europepmc.org/article/NBK/nbk564350
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87(5):779–789. https://doi.org/10.1189/jlb.1109766
Mo Y, Sun YY, Liu KY (2020) Autophagy and inflammation in ischemic stroke. Neural Regen Res 15(8):1388–1396. https://doi.org/10.4103/1673-5374.274331
Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG (2006) Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke 37(4):1087–1093. https://doi.org/10.1161/01.STR.0000206281.77178.ac
Liesz A, Hu X, Kleinschnitz C, Offner H (2015) Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke 46(5):1422–1430. https://doi.org/10.1161/STROKEAHA.114.008608
Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA (2019) Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation 16(1):142. https://doi.org/10.1186/s12974-019-1516-2
Candelario-Jalil E, Dijkhuizen RM, Magnus T (2022) Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke 53(5):1473–1486. https://doi.org/10.1161/STROKEAHA.122.036946
Zhang S, Lachance BB, Moiz B, Jia X (2020) Optimizing stem cell therapy after ischemic brain injury. J Stroke 22(3):286–305. https://doi.org/10.5853/jos.2019.03048
Li W, Shi L, Hu B, Hong Y, Zhang H, Li X, Zhang Y (2021) Mesenchymal stem cell-based therapy for stroke: current understanding and challenges. Front Cell Neurosci 15:628940. https://doi.org/10.3389/fncel.2021.628940
Li X, Zhang X, Liu Y, Pan R, Liang X, Huang L, Yang C (2021) Exosomes derived from mesenchyml stem cells ameliorate oxygen-glucose deprivation/reoxygenation-induced neuronal injury via transferring MicroRNA-194 and targeting Bach1. Tissue Cell 73:101651. https://doi.org/10.1016/j.tice.2021.101651
Pant T, Juric M, Bosnjak ZJ, Dhanasekaran A (2021) Recent insight on the non-coding RNAs in Mesenchymal stem cell-derived exosomes: regulatory and therapeutic role in regenerative medicine and tissue engineering. Front Cardiovasc Med 8:737512. https://doi.org/10.3389/fcvm.2021.737512
Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F (2018) MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 17(21–22):2411–2422. https://doi.org/10.1080/15384101.2018.1526603
Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX (2019) LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther 10(1):393. https://doi.org/10.1186/s13287-019-1522-4
Cheng M, Liu L, Zhang T, Chen Y, Wang Q, Wu Y (2022) Extracellular vesicles derived from bone marrow mesenchymal stem cells alleviate neurological deficit and endothelial cell dysfunction after subarachnoid hemorrhage via the KLF3-AS1/miR-83-5p/TCF7L2 axis. Exp Neurol 356:114151. https://doi.org/10.1016/j.expneurol.2022.114151
Han ZF, Cao JH, Liu ZY, Yang Z, Qi RX, Xu HL (2022) Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Pract 183:109126. https://doi.org/10.1016/j.diabres.2021.109126
Chen G, Yue A, Wang M, Ruan Z, Zhu L (2021) The exosomal lncRNA KLF3-AS1 from ischemic cardiomyocytes mediates IGF-1 secretion by MSCs to rescue myocardial ischemia-reperfusion injury. Front Cardiovasc Med 8:671610. https://doi.org/10.3389/fcvm.2021.671610
Wen C, Lin L, Zou R, Lin F, Liu Y (2022) Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell Cycle 21(3):289–303. https://doi.org/10.1080/15384101.2021.2019411
Xie X, Cao Y, Dai L, Zhou D (2023) Bone marrow mesenchymal stem cell-derived exosomal lncRNA KLF3-AS1 stabilizes Sirt1 protein to improve cerebral ischemia/reperfusion injury via miR-206/USP22 axis. Mol Med 29(1):3. https://doi.org/10.1186/s10020-022-00595-1
Zhou F, Wang YK, Zhang CG, Wu BY (2021) miR-19a/b-3p promotes inflammation during cerebral ischemia/reperfusion injury via SIRT1/FoxO3/SPHK1 pathway. J Neuroinflammation 18(1):122. https://doi.org/10.1186/s12974-021-02172-5
Moon E, Han JE, Jeon S, Ryu JH, Choi JW, Chun J (2015) Exogenous S1P exposure potentiates ischemic stroke damage that is reduced possibly by inhibiting S1P receptor signaling. Mediators Inflamm 2015:492659. https://doi.org/10.1155/2015/492659
Zheng S, Wei S, Wang X, Xu Y, Xiao Y, Liu H, Jia J, Cheng J (2015) Sphingosine kinase 1 mediates neuroinflammation following cerebral ischemia. Exp Neurol 272:160–169. https://doi.org/10.1016/j.expneurol.2015.03.012
Su D, Cheng Y, Li S, Dai D, Zhang W, Lv M (2017) Sphk1 mediates neuroinflammation and neuronal injury via TRAF2/NF-kappaB pathways in activated microglia in cerebral ischemia reperfusion. J Neuroimmunol 305:35–41. https://doi.org/10.1016/j.jneuroim.2017.01.015
Lv M, Zhang D, Dai D, Zhang W, Zhang L (2016) Sphingosine kinase 1/sphingosine-1-phosphate regulates the expression of interleukin-17A in activated microglia in cerebral ischemia/reperfusion. Inflamm Res 65(7):551–562. https://doi.org/10.1007/s00011-016-0939-9
Horisawa K, Imai T, Okano H, Yanagawa H (2010) The Musashi family RNA-binding proteins in stem cells. Biomol Concepts 1(1):59–66. https://doi.org/10.1515/bmc.2010.005
Padial-Molina M, de Buitrago JG, Sainz-Urruela R, Abril-Garcia D, Anderson P, O'Valle F, Galindo-Moreno P (2019) Expression of Musashi-1 during osteogenic differentiation of oral MSC: an in vitro study. Int J Mol Sci 20(9). https://doi.org/10.3390/ijms20092171
Good P, Yoda A, Sakakibara S, Yamamoto A, Imai T, Sawa H, Ikeuchi T, Tsuji S et al (1998) The human Musashi homolog 1 (MSI1) gene encoding the homologue of Musashi/Nrp-1, a neural RNA-binding protein putatively expressed in CNS stem cells and neural progenitor cells. Genomics 52(3):382–384. https://doi.org/10.1006/geno.1998.5456
Zhang XC, Liang HF, Luo XD, Wang HJ, Gu AP, Zheng CY, Su QZ, Cai J (2018) YY1 promotes IL-6 expression in LPS-stimulated BV2 microglial cells by interacting with p65 to promote transcriptional activation of IL-6. Biochem Biophys Res Commun 502(2):269–275. https://doi.org/10.1016/j.bbrc.2018.05.159
Peng LS, Xu Y, Wang QS (2022) Yy1 promotes microglia M2 polarization through the Mir-130a-3p/Trem-2 axis to alleviate sepsis-associated encephalopathy. Shock 58(2):128–136. https://doi.org/10.1097/SHK.0000000000001914
Lu J, Jin K, Jiao J, Liu R, Mou T, Chen B, Zhang Z, Jiang C et al (2023) YY1 (Yin-Yang 1), a transcription factor regulating systemic inflammation, is involved in cognitive impairment of depression. Psychiatry Clin Neurosci 77(3):149–159. https://doi.org/10.1111/pcn.13510
Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F (2019) Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/beta-catenin pathway. Stem Cell Res Ther 10(1):98. https://doi.org/10.1186/s13287-019-1204-2
Wang JJ, Ye F, Cheng LJ, Shi YJ, Bao J, Sun HQ, Wang W, Zhang P et al (2009) Osteogenic differentiation of mesenchymal stem cells promoted by overexpression of connective tissue growth factor. J Zhejiang Univ Sci B 10(5):355–367. https://doi.org/10.1631/jzus.B0820252
Li YJ, Xu QW, Xu CH, Li WM (2022) MSC promotes the secretion of exosomal miR-34a-5p and improve intestinal barrier function through METTL3-mediated pre-miR-34A m(6)a modification. Mol Neurobiol 59(8):5222–5235. https://doi.org/10.1007/s12035-022-02833-3
Song MY, Yi F, Xiao H, Yin J, Huang Q, Xia J, Yin XM, Wen YB et al (2022) Energy restriction induced SIRT6 inhibits microglia activation and promotes angiogenesis in cerebral ischemia via transcriptional inhibition of TXNIP. Cell Death Dis 13(5):449. https://doi.org/10.1038/s41419-022-04866-x
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91. https://doi.org/10.1161/01.str.20.1.84
Zuo G, Zhang D, Mu R, Shen H, Li X, Wang Z, Li H, Chen G (2018) Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats. Mol Brain 11(1):9. https://doi.org/10.1186/s13041-018-0351-1
Cheiloudaki E, Alexopoulos EC (2019) Adherence to treatment in stroke patients. Int J Environ Res Public Health 16(2). https://doi.org/10.3390/ijerph16020196
Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC et al (2003) Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res 73(6):778–786. https://doi.org/10.1002/jnr.10691
Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH et al (2005) Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49(3):407–417. https://doi.org/10.1002/glia.20126
Bryan L, Kordula T, Spiegel S, Milstien S (2008) Regulation and functions of sphingosine kinases in the brain. Biochim Biophys Acta 1781(9):459–466. https://doi.org/10.1016/j.bbalip.2008.04.008
Pawluk H, Wozniak A, Grzesk G, Kolodziejska R, Kozakiewicz M, Kopkowska E, Grzechowiak E, Kozera G (2020) The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin Interv Aging 15:469–484. https://doi.org/10.2147/CIA.S233909
Chauveau F, Cho TH, Berthezene Y, Nighoghossian N, Wiart M (2010) Imaging inflammation in stroke using magnetic resonance imaging. Int J Clin Pharmacol Ther 48(11):718–728. https://doi.org/10.5414/cpp48718
Shen XY, Gao ZK, Han Y, Yuan M, Guo YS, Bi X (2021) Activation and role of astrocytes in ischemic stroke. Front Cell Neurosci 15:755955. https://doi.org/10.3389/fncel.2021.755955
Wang H, Song G, Chuang H, Chiu C, Abdelmaksoud A, Ye Y, Zhao L (2018) Portrait of glial scar in neurological diseases. Int J Immunopathol Pharmacol 31:2058738418801406. https://doi.org/10.1177/2058738418801406
Forouzanfar M, Lachinani L, Dormiani K, Nasr-Esfahani MH, Gure AO, Ghaedi K (2020) Intracellular functions of RNA-binding protein, Musashi1, in stem and cancer cells. Stem Cell Res Ther 11(1):193. https://doi.org/10.1186/s13287-020-01703-w
Verheul TCJ, van Hijfte L, Perenthaler E, Barakat TS (2020) The why of YY1: mechanisms of transcriptional regulation by Yin Yang 1. Front Cell Dev Biol 8:592164. https://doi.org/10.3389/fcell.2020.592164
Chen YH, Chung CC, Liu YC, Lai WC, Lin ZS, Chen TM, Li LY, Hung MC (2018) YY1 and HDAC9c transcriptionally regulate p38-mediated mesenchymal stem cell differentiation into osteoblasts. Am J Cancer Res 8(3):514–525
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding
This work was supported by National Natural Science Foundation of China (82101540) and Scientifc research project of Hunan Provincial Health Commission (B202304049105, D202314056910).
Author information
Authors and Affiliations
Contributions
Yu Cao: Conceptualization, Methodology, Writing- Original draft preparation, Investigation, Validation, Visualization.
Daodao Wang: Data curation, Software.
Dingzhou Zhou: Conceptualization, Writing- Original draft preparation, Supervision, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The animal study was approved by Hunan Provincial People’s Hospital (The first-affiliated hospital of Hunan normal university).
Conflict of Interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, Y., Wang, D. & Zhou, D. MSC Promotes the Secretion of Exosomal lncRNA KLF3-AS1 to Regulate Sphk1 Through YY1-Musashi-1 Axis and Improve Cerebral Ischemia–Reperfusion Injury. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04150-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04150-3