Abstract
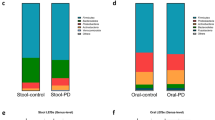
Cognitive impairment (CI) is a common complication of the non-motor symptoms in Parkinson’s disease (PD), including PD with mild cognitive impairment (PD-MCI) and PD dementia. Recent studies reported the oral dysbiosis in PD and CI, respectively. Porphyromonas gingivalis (P. gingivalis), a pathogen of oral dysbiosis, plays an important role in PD, whose lysine-gingipain (Kgp) could lead to AD-type pathologies. No previous study investigated the composition of oral microbiota and role of P. gingivalis in PD-MCI. This study aimed to investigate the differences of oral microbiota composition, P. gingivalis copy number, and Kgp genotypes among PD-MCI, PD with normal cognition (PD-NC) and periodontal status-matched control (PC) groups. The oral bacteria composition, the copy number of P. gingivalis, and the Kgp genotypes in gingival crevicular fluid from PD-MCI, PD-NC, and PC were analyzed using 16S ribosomal RNA sequencing, quantitative real-time PCR, and MseI restriction. We found that the structures of oral microbiota in PD-MCI group were significantly different compared to that in PD-NC and PC group. The relative abundances of Prevotella, Lactobacillus, Megasphaera, Atopobium, and Howardella were negatively correlated with cognitive score. Moreover, there was a significant difference of Kgp genotypes among the three groups. The predominant Kgp genotypes of P. gingivalis in the PD-MCI group were primarily Kgp II, whereas in the PD-NC group, it was mainly Kgp I. The Kgp II correlated with lower MMSE and MoCA scores, which suggested that Kgp genotypes II is related to cognitive impairment in PD.


Similar content being viewed by others
Data Availability
Raw sequencing data was deposited in the NCBI GenBank under BioProject ID PRJNA1030740.
References
Hoogland J, Boel JA, de Bie RMA, Geskus RB, Schmand BA, Dalrymple-Alford JC, Marras C, Adler CH et al (2017) Mild cognitive impairment as a risk factor for Parkinson’s disease dementia. Movement Disord 32(7):1056–1065. https://doi.org/10.1002/mds.27002
Pedersen KF, Larsen JP, Tysnes O, Alves G (2017) Natural course of mild cognitive impairment in Parkinson disease. Neurology 88(8):767–774. https://doi.org/10.1212/WNL.0000000000003634
Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, Collins C, Lashley T, Kallis C et al (2011) Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain 134(5):1493–1505. https://doi.org/10.1093/brain/awr031
Irwin DJ, Lee VMY, Trojanowski JQ (2013) Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci 14(9):626–636. https://doi.org/10.1038/nrn3549
Pereira PAB, Aho VTE, Paulin L, Pekkonen E, Auvinen P, Scheperjans F (2017) Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat D 38:61–67. https://doi.org/10.1016/j.parkreldis.2017.02.026
Fleury V, Zekeridou A, Lazarevic V, Gaïa N, Giannopoulou C, Genton L, Cancela J, Girard M et al (2021) Oral dysbiosis and inflammation in Parkinson’s disease. J Parkinsons Dis 11(2):619–631. https://doi.org/10.3233/JPD-202459
Yay E, Yilmaz M, Toygar H, Balci N, Alvarez Rivas C, Bolluk Kilic B, Zirh A, Paster B et al (2023) Parkinson’s disease alters the composition of subgingival microbiome. J Oral Microbiol 15(1). https://doi.org/10.1080/20002297.2023.2250650
Na HS, Jung NY, Song Y, Kim SY, Kim HJ, Lee JY, Chung J (2023) A distinctive subgingival microbiome in patients with periodontitis and Alzheimer’s disease compared with cognitively unimpaired periodontitis patients. J Clin Periodontol 51(1):43–53. https://doi.org/10.1111/jcpe.13880
Adams B, Nunes JM, Page MJ, Roberts T, Carr J, Nell TA, Kell DB, Pretorius E (2019) Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Aging Neurosci 11:210. https://doi.org/10.3389/fnagi.2019.00210
Chen C, Wu Y, Chang Y (2017) Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: a population-based retrospective matched-cohort study. Peerj 5:e3647. https://doi.org/10.7717/peerj.3647
Jeong E, Park J, Park Y (2021) Evaluation of the association between periodontitis and risk of Parkinson’s disease: a nationwide retrospective cohort study. Sci Rep-Uk 11(1):16594. https://doi.org/10.1038/s41598-021-96147-4
Feng Y, Wu Q, Peng Y, Liang F, You H, Feng Y, Li G, Li X et al (2020) Oral P. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J Neuroinflamm 17(1):1–12. https://doi.org/10.1186/s12974-020-02027-5
Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, Fuller J, Ibbett P et al (2016) Periodontitis and cognitive decline in Alzheimer’s disease. Plos One 11(3):e151081. https://doi.org/10.1371/journal.pone.0151081
Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik Sobanska L, Leon MJ (2008) Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement 4(4):242–250. https://doi.org/10.1016/j.jalz.2007.08.004
Chen C, Wu Y, Chang Y (2017) Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimers Res Ther 9(1):1–7. https://doi.org/10.1186/s13195-017-0282-6
Noble JM, Borrell LN, Papapanou PN, Elkind MSV, Scarmeas N, Wright CB (2009) Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry 80(11):1206–1211. https://doi.org/10.1136/jnnp.2009.174029
Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, Dawson D (2012) Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement 8(3):196–203. https://doi.org/10.1016/j.jalz.2011.04.006
Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S (2013) Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimer's Dis 36(4):665–677. https://doi.org/10.3233/JAD-121918
Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U et al (2019) Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5(1):u3333. https://doi.org/10.1126/sciadv.aau3333
Beikler T, Peters U, Ehmke B, Flemmig TF (2003) Sequence analysis of kgp in Porphyromonas gingivalis isolates from periodontitis patients. Oral Microbiol Immunol 18(6):393–397. https://doi.org/10.1046/j.0902-0055.2003.00106.x
Li D, Ren T, Li H, Liao G, Zhang X (2022) Porphyromonas gingivalis: a key role in Parkinson’s disease with cognitive impairment? Front Neurol 13:945523. https://doi.org/10.3389/fneur.2022.945523
Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ (1992) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 42(6):1142–1146. https://doi.org/10.1212/wnl.42.6.1142
Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Movement Disord 27(3):349–356. https://doi.org/10.1002/mds.24893
Manoil D, Bostanci N, Mumcu G, Inanc N, Can M, Direskeneli H, Belibasakis GN (2021) Novel and known periodontal pathogens residing in gingival crevicular fluid are associated with rheumatoid arthritis. J Periodontol 92(3):359–370. https://doi.org/10.1002/JPER.20-0295
Ren T, Gao Y, Qiu Y, Jiang S, Zhang Q, Zhang J, Wang L, Zhang Y et al (2020) Gut microbiota altered in mild cognitive impairment compared with normal cognition in sporadic Parkinson’s disease. Front Neurol 11:504772. https://doi.org/10.3389/fneur.2020.00137
Aljumaah MR, Bhatia U, Roach J, Gunstad J, Azcarate Peril MA (2022) The gut microbiome, mild cognitive impairment, and probiotics: a randomized clinical trial in middle-aged and older adults. Clin Nutr 41(11):2565–2576. https://doi.org/10.1016/j.clnu.2022.09.012
Khedr EM, Omeran N, Karam-Allah Ramadan H, Ahmed GK, Abdelwarith AM (2022) Alteration of gut microbiota in Alzheimer’s disease and their relation to the cognitive impairmenT. J Alzheimer's Dis 88(3):1103–1114. https://doi.org/10.3233/JAD-220176
Wallen ZD, Appah M, Dean MN, Sesler CL, Factor SA, Molho E, Zabetian CP, Standaert DG et al (2020) Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis 6:11. https://doi.org/10.1038/s41531-020-0112-6
Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S et al (2015) Gut microbiota are related to Parkinson’s disease and clinical phenotype. Movement Disord 30(3):350–358. https://doi.org/10.1002/mds.26069
Jo S, Kang W, Hwang YS, Lee SH, Park KW, Kim MS, Lee H, Yoon HJ et al (2022) Oral and gut dysbiosis leads to functional alterations in Parkinson’s disease. NPJ Parkinsons Dis 8(1):87. https://doi.org/10.1038/s41531-022-00351-6
Toh TS, Chong CW, Lim S, Bowman J, Cirstea M, Lin C, Chen C, Appel-Cresswell S et al (2022) Gut microbiome in Parkinson’s disease: new insights from meta-analysis. Parkinsonism Relat D 94:1–9. https://doi.org/10.1016/j.parkreldis.2021.11.017
Pessione E (2012) Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Mi 2:86. https://doi.org/10.3389/fcimb.2012.00086
Filosa S, Di Meo F, Crispi S (2018) Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen Res 13(12):2055–2059. https://doi.org/10.4103/1673-5374.241429
Nie R, Wu Z, Ni J, Zeng F, Yu W, Zhang Y, Kadowaki T, Kashiwazaki H et al (2019) Porphyromonas gingivalis infection induces amyloid-β accumulation in monocytes/macrophages. J Alzheimer's Dis 72(2):479–494. https://doi.org/10.3233/JAD-190298
Kim S, Kwon S, Kam T, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR et al (2019) Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103(4):627–641. https://doi.org/10.1016/j.neuron.2019.05.035
Funding
This work was supported by grants from the National Natural Science Foundation of China (81360198), the Special Fund for Science and Technology Innovation Strategy of Guangdong province (grant number 2021S0025, 2021S0026, and 2021S0031), and Maoming Science and Technology Special Fund Plan and Project (grant number 2020KJZX006). XZ was supported by the Doctoral Research Foundation of Maoming People’s Hospital.
Author information
Authors and Affiliations
Contributions
Xiong Zhang and Renshi Xu conceptualized and designed the study. Dongcheng Li, Tengzhu Ren, and Hao Li organize the researches. Dongcheng Li, Mingdi Huang, Jiaxin Chen, and Qishan He performed data collection and analysis. Dongcheng Li, Qishan He, and Tengzhu Ren analyzed and interpreted data. Dongcheng Li wrote the manuscript draft. Xiong Zhang and Tengzhu Ren contributed to the drafting and critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The studies involving human participants were reviewed and approved by Ethics Committee of Maoming People’s Hospital (reference No. PJ2022MI-K011-01).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, D., Ren, T., Li, H. et al. Oral Microbiota and Porphyromonas gingivalis Kgp Genotypes Altered in Parkinson’s Disease with Mild Cognitive Impairment. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04119-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04119-2