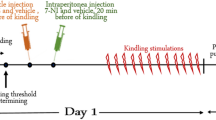
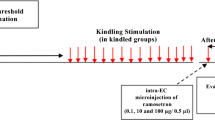
Abstract
Epilepsy is characterized by a sustained depolarization and repeated discharge of neurons, attributed to overstimulation of N-methyl-D-aspartate receptors (NMDAr). Herein, we propose that probenecid (PROB), an inhibitor of the activity of some ATP binding-cassette transporters (ABC-transporters) can modify NMDAr activity and expression in amygdaloid kindled model. Some studies have suggested that NMDAr expression could be regulated by inhibiting the activity of P-glycoprotein (MDR1) and drug resistance protein-1 (MRP1). Besides, PROB was found to interact with other proteins with proven activity in the kindling model, such as TRPV2 channels, OAT1, and Panx1. Administering PROB at two doses (100 and 300 mg/kg/d) for 5 d decreased after-discharge duration and Racine behavioral scores. It also reduced the expression of NR2B and the activity of total NOS and the expression of nNOS with respect to the kindling group. In a second protocol, voltage-clamp measurements of NMDA-evoked currents were performed in CA1 hippocampal cells dissociated from control and kindled rats. PROB produced a dose-dependent reduction in NMDA-evoked currents. In neurons from kindled rats, a residual NMDA-evoked current was registered with respect to control animals, while a reduction in NMDA-evoked currents was observed in the presence of 20 mM PROB. Finally, we evaluated the expression of MRP1 and MDR1 in order to establish a relationship between the reduction of kindling parameters, the inhibition of NMDA-type currents, and the expression of these transporters. Based on our results, we conclude that at the concentrations used, PROB inhibits currents evoked by NMDA in dissociated neurons of control and kindled rats. In the kindling model, at the tested doses, PROB decreases the after-discharge duration and Racine behavioral score in the kindling model. We propose a mechanism that could be dependent on the expression of ABC-type transporters.







Similar content being viewed by others
Data Availability
The datasets used and/or analyses during the current study are available from the corresponding author upon reasonable request.
References
MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL (1986) NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321(6069):519–522
Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325(6104):529–531
Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307(5950):462–465
Mayer ML, Westbrook GL, Guthrie PB (1984) Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309(5965):261–263
Collingridge GL, Bliss TV (1995) Memories of NMDA receptors and LTP. Trends Neurosci 18(2):54–56
Bliss TV, Collingridge GL (2013) Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol Brain 6:5
Mody I, Heinemann U (1987) NMDA receptors of dentate gyrus granule cells participate in synaptic transmission following kindling. Nature 326(6114):701–4
Sprengel R, Suchanek B, Amico C et al (1998) Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell 92(2):279–289
Bankstahl JP, Hoffmann K, Bethmann K, Loscher W (2008) Glutamate is critically involved in seizure-induced overexpression of P-glycoprotein in the brain. Neuropharmacology 54(6):1006–1016
Avemary J, Salvamoser JD, Peraud A et al (2013) Dynamic regulation of P-glycoprotein in human brain capillaries. Mol Pharm 10(9):3333–3341
Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM, Raffel C (1995) MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia 36(1):1–6
Smolarz B, Makowska M, Romanowicz H (2021) Pharmacogenetics of Drug-Resistant Epilepsy (Review of Literature). Int J Mol Sci 22(21):11696
Lazarowski A, Sevlever G, Taratuto A, Massaro M, Rabinowicz A (1999) Tuberous sclerosis associated with MDR1 gene expression and drug-resistant epilepsy. Pediatr Neurol 21(4):731–734
Sisodiya SM, Heffernan J, Squier MV (1999) Over-expression of P-glycoprotein in malformations of cortical development. NeuroReport 10(16):3437–3441
Dombrowski SM, Desai SY, Marroni M et al (2001) Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 42(12):1501–1506
Lazarowski A, Lubieniecki F, Camarero S et al (2004) Multidrug resistance proteins in tuberous sclerosis and refractory epilepsy. Pediatr Neurol 30(2):102–106
Lazarowski A, Massaro M, Schteinschnaider A, Intruvini S, Sevlever G, Rabinowicz A (2004) Neuronal MDR-1 gene expression and persistent low levels of anticonvulsants in a child with refractory epilepsy. Ther Drug Monit 26(1):44–46
Zhu HJ, Liu GQ (2004) Glutamate up-regulates P-glycoprotein expression in rat brain microvessel endothelial cells by an NMDA receptor-mediated mechanism. Life Sci 75(11):1313–1322
Yang T, Kong B, Kuang Y et al (2015) Emodin plays an interventional role in epileptic rats via multidrug resistance gene 1 (MDR1). Int J Clin Exp Pathol 8(3):3418–3425
Cheng MH, Kim SJ (2020) Inhibitory Effect of Probenecid on Osteoclast Formation via JNK, ROS and COX-2. Biomol Ther (Seoul) 28(1):104–109
Du L, Empey PE, Ji J et al (2016) Probenecid and N-Acetylcysteine Prevent Loss of Intracellular Glutathione and Inhibit Neuronal Death after Mechanical Stretch Injury In Vitro. J Neurotrauma 33(20):1913–1917
Jiang B, Zhao Y, Cao J et al (2021) Synthesis and preliminary biological evaluation of naproxen-probenecid conjugate for central nervous system (CNS) delivery. Pak J Pharm Sci 34(6):2197–2203
Vamos E, Voros K, Zadori D, Vecsei L, Klivenyi P (2009) Neuroprotective effects of probenecid in a transgenic animal model of Huntington’s disease. J Neural Transm (Vienna) 116(9):1079–1086
Bang S, Kim KY, Yoo S, Lee SH, Hwang SW (2007) Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett 425:120–125
SilvermanW LS, Dahl G (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295:C761–C767
Taylor DL, Urenjak J, Zilkha E, Obrenovitch TP (1997) Effects of probenecid on the elicitation of spreading depression in the rat striatum. Brain Res 764(1–2):117–125
Urenjak J, Obrenovitch TP, Zilkha E (1997) Effect of probenecid on depolarizations evoked by N-methyl-D-aspartate (NMDA) in the rat striatum. Naunyn Schmiedebergs Arch Pharmacol 355(1):36–42
Paxinos G, Watson CH (2013) The Rat Brain in Stereotaxic Coordinates, 7th edn. Academic Press, New York
Hernandez-Ceron M, Martinez-Lazcano JC, Rubio C et al (2017) Participation of the dentate-rubral pathway in the kindling model of epilepsy. J Neurosci Res 95(7):1495–1502
Goddard GV, McIntyre DC, Leech CK (1969) A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 25(3):295–330
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32(3):281–94
Martinez-Lazcano JC, Gonzalez-Guevara E, Custodio V et al (2018) Activity of nitric oxide synthase isoforms in acute brain oxidative damage induced by ozone exposure. Nitric Oxide 75:42–52
Martinez-Lazcano JC, Perez-Severiano F, Escalante B et al (2007) Selective protection against oxidative damage in brain of mice with a targeted disruption of the neuronal nitric oxide synthase gene. J Neurosci Res 85(7):1391–1402
Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ (1994) Cellular and molecular characterization of Ca2+ currents in acutely isolated, adult rat neostriatal neurons. J Neurosci 14(11 Pt 1):6667–6686
Flores-Hernandez J, Galarraga E, Pineda JC, Bargas J (1994) Patterns of excitatory and inhibitory synaptic transmission in the rat neostriatum as revealed by 4-AP. J Neurophysiol 72(5):2246–2256
Rendon-Ochoa EA, Hernandez-Flores T, Aviles-Rosas VH et al (2018) Calcium currents in striatal fast-spiking interneurons: dopaminergic modulation of CaV1 channels. BMC Neurosci 19(1):42
Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E et al (2002) Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol 88(6):3010–3020
Campos-Arroyo D, Maldonado V, Bahena I et al (2016) Probenecid Sensitizes Neuroblastoma Cancer Stem Cells to Cisplatin. Cancer Invest 34(3):155–66
Leino E, MacDonald E, Airaksinen MM, Riekkinen PJ (1980) Homovanillic acid and 5-hydroxyindoleacetic acid levels in cerebrospinal fluid of patients with progressive myoclonus epilepsy. Acta Neurol Scand 62(1):41–54
Shaywitz BA, Cohen DJ, Leckman JF, Young JG, Bowers MB (1980) Ontogeny of dopamine and serotonin metabolites in the cerebrospinal fluid of children with neurological disorders. Dev Med Child Neurol 22(6):748–754
Fukuyama Y, Ochiai Y (1982) Therapeutic trial by taurine for intractable childhood epilepsies. Brain Dev 4(1):63–69
Tang CM, Dichter M, Morad M (1990) Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci U S A 87(16):6445–6449
Lipton SA, Rosenberg PA (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330(9):613–622
West AE, Chen WG et al (2001) Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A 98(20):11024–11031
Banach M, Piskorska B, Czuczwar SJ, Borowicz KK (2011) Nitric oxide, epileptic seizures, and action of antiepileptic drugs. CNS Neurol Disord Drug Targets 10(7):808–819
Bankstahl JP, Kuntner C, Abrahim A et al (2008) Langer, Tariquidar-induced P-glycoprotein inhibition at the rat blood-brain barrier studied with (R)-11C-verapamil and PET. J Nucl Med 49(8):1328–1335
Sutula TP (2004) Mechanisms of epilepsy progression: current theories and perspectives from neuroplasticity in adulthood and development. Epilepsy Res 60(2–3):161–171
Sutula TP, Dudek FE (2007) Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res 163:541–563
McNamara JO (1995) Analyses of the molecular basis of kindling development. Psychiatry Clin Neurosci 49(3):S175–S178
Kohr G, De Koninck Y, Mody I (1993) Properties of NMDA receptor channels in neurons acutely isolated from epileptic (kindled) rats. J Neurosci 13(8):3612–3627
Behr J, Heinemann U, Mody I (2000) Glutamate receptor activation in the kindled dentate gyrus. Epilepsia 41(Suppl 6):S100–S103
Kamphuis W, Monyer H, De Rijk TC, Lopes da Silva FH (1992) Hippocampal kindling increases the expression of glutamate receptor-A Flip and -B Flip mRNA in dentate granule cells. Neurosci Lett 148(1–2):51–54
Chen YH, Wang CC, Xiao X, Wei L, Xu G (2013) Multidrug resistance-associated protein 1 decreases the concentrations of antiepileptic drugs in cortical extracellular fluid in amygdale kindling rats. Acta Pharmacol Sin 34(4):473–479
Bauer B, Hartz AM, Pekcec A, Toellner K, Miller DS, Potschka H (2008) Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol 73(5):1444–1453
García-Rodríguez C, Mujica P, Illanes-González J, López A, Vargas C et al (2023) Probenecid, an Old Drug with Potential New Uses for Central Nervous System Disorders and Neuroinflammation. Biomedicines 11(6):1516
Acknowledgements
The authors would like to thank Dr. Carlos Paz Tres for facilitating the infrastructure of his laboratory, Dr. Denise Campos-Arroyo for her supervision in the design and development of the semi-quantitative RT-PCR technique, Francisco Gutiérrez-Baeza by his technical support, and Juan Francisco Rodriguez for copyediting the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed sufficiently for being credited as an author of this article. Conception and design: JCML, JFP and VC. JFP and MHC performed the stereotaxic surgery and the development of the kindling model. EGG and CMS performed the molecular experiments. ELG, ERO, and AL and JB performed the patch clamp experiment and data analysis. FPS performed the enzymatic activity experiment. VC provided technical support. EGG, ELG, and JCML performed data analysis. The first draft of the manuscript was written by JCML and all authors contributed to previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was conducted in accordance with the institutional guidelines and national regulation (NOM-062-ZOO-1999) from the Guide for the Care and Use of Laboratory Animals (8th edition), and international guiding principles (Council for International Organizations of Medical Sciences, CIOMS)
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests or financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
González-Guevara, E., Lara-González, E., Rendon-Ochoa, E. et al. Inhibition of the NMDA Currents by Probenecid in Amygdaloid Kindling Epilepsy Model. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03969-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03969-0