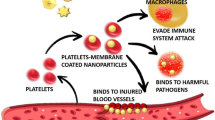
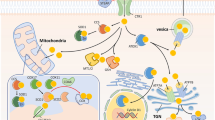
Abstract
Wilson disease, a rare genetic disorder resulting from mutations in the ATP7B gene disrupts copper metabolism, leading to its harmful accumulation in hepatocytes, the brain, and other organs. It affects roughly 1 in 30,000 individuals, with 1 in 90 being gene carriers. Beyond gene mutations, the disease involves complex factors contributing to copper imbalance. Ongoing research seeks to unravel intricate molecular pathways, offering fresh insights into the disease’s mechanisms. Simultaneously, there is a dedicated effort to develop effective therapeutic strategies. Nanotechnology-driven formulations are showing promise for both treatment and early diagnosis of Wilson disease. This comprehensive review covers the entire spectrum of the condition, encompassing pathophysiology, potential biomarkers, established and emerging therapies, ongoing clinical trials, and innovative nanotechnology applications. This multifaceted approach holds the potential to improve our understanding, diagnosis, and management of Wilson’s disease, which remains a challenging and potentially life-threatening disorder.
Graphical Abstract




Similar content being viewed by others
Data Availability
The review article-related raw data files/figures/tables are available from the corresponding author.
Abbreviations
- 2-OG:
-
2-Oxoglutarate
- 8-OHdG:
-
8-Hydroxy-2′-deoxyguanosine
- AAV:
-
Adeno-associated virus
- ATOX-1:
-
Human antioxidant protein 1
- ATP:
-
Adenosine triphosphate
- ATP7A:
-
Cu(I) transporting ATPase A
- ATP7B :
-
Cu(I) transporting ATPase B
- BBB:
-
Blood-brain barrier
- CCS:
-
Copper chaperon for superoxide dismutase
- CHE:
-
Pseudocholinesterase
- COMMD1:
-
Copper metabolism domain-containing 1 protein
- COX:
-
Cytochrome c oxidase assembly protein
- Cp:
-
Ceruloplasmin
- CTR-1:
-
Copper-transporting protein-1
- CTR2:
-
Copper-transporting protein-2
- Cu:
-
Copper
- DMSA:
-
Dimercaptosuccinic acid
- EASL:
-
European Association for the Study of the Liver
- GDL:
-
GanDouLing
- GFAP:
-
Glial fibrillary acidic protein
- GSH:
-
Glutathione
- LEA:
-
Long-Evans Agouti rat model
- LEC:
-
Long-Evans Cinnamon rat model
- MiADMSA:
-
Monoisoamyl 2,3-dimercaptosuccinic acid
- MTs:
-
Copper-binding metallothioneins
- NCC:
-
Non-caeruloplasmin-bound copper
- NfL:
-
Neurofilament
- SAM:
-
S-adenosyl-methionine
- Sco 1/2:
-
Cytochrome c oxidase subunits 1 and 2
- SDH:
-
Succinate dehydrogenase
- SHED:
-
Stem cells from human-exfoliated deciduous teeth
- SHED-Heps:
-
SHED-converted hepatocyte-like cells
- SOD1:
-
Superoxide dismutase 1
- SREBP-2:
-
Sterol regulatory-binding protein 2
- TGN:
-
Trans-Golgi network
- TTM:
-
Tetrathiomolybdate
- Tx-j:
-
Jackson’s toxic milk mouse model
- UCH-L1:
-
Ubiquitin carboxyl-terminal hydrolase L1
- WD:
-
Wilson’s disease
- WGBS:
-
Whole-genome bisulfite sequencing
- XIAP:
-
X-linked inhibitor of apoptosis protein
References
Arnal N, de Alaniz MJT, Marra CA (2012) Cytotoxic effects of copper overload on human-derived lung and liver cells in culture. Elsevier. https://doi.org/10.1016/j.bbagen.2012.03.007
Patwa J, Thakur A, Sharma A, Flora SJS (2020) Monoisoamyl DMSA reduced copper-induced neurotoxicity by lowering 8-OHdG level, amyloid beta and Tau protein expressions in Sprague-Dawley rats. Metallomics 12:1428–1448. https://doi.org/10.1039/D0MT00083C
Chaudhry H, Anilkumar A (2023) Wilson disease
Hepatology EAFTSOTL-J of (2012) EASL clinical practice guidelines: Wilson’s disease. Elsevier 56:671–685
Netter P, Bannwarth B, Péré P, Nicolas A (1987) Clinical pharmacokinetics of D-penicillamine. Clin Pharmacokinet 13:317–333. https://doi.org/10.2165/00003088-198713050-00003
Li W-J, Chen C, You Z-F et al (2016) Current drug managements of Wilson’s disease: from west to east. Curr Neuropharmacol 14:322. https://doi.org/10.2174/1570159X14666151130222427
Aronson J (2014) Meyler’s side effects of drugs 15E: the International Encyclopedia of Adverse Drug Reactions and Interactions
Rosenberg RN, Pascual JM (2020) Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease, 6th ed. Volume 1. Elsevier
Merle U, Schaefer M, Ferenci P, Stremmel W (2007) Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut 56:115–120. https://doi.org/10.1136/gut.2005.087262
Mhaske A, Sharma S, RS-J of DDS (2023) Nanotheranostic: the futuristic therapy for copper mediated neurological sequelae. Elsevier, pp. 1773–2247
Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY (2007) Function and regulation of human copper-transporting ATPases. Physiol Rev 87:1011–1046. https://doi.org/10.1152/PHYSREV.00004.2006
Dmitriev O, Tsivkovskii R, Abildgaard F et al (2006) Solution structure of the N-domain of Wilson disease protein: distinct nucleotide-binding environment and effects of disease mutations. Proc Natl Acad Sci USA 103:5302–5307. https://doi.org/10.1073/PNAS.0507416103
Møller LB, Horn N, Jeppesen TD et al (2011) Clinical presentation and mutations in Danish patients with Wilson disease. Eur J Hum Genet 19:935–941. https://doi.org/10.1038/ejhg.2011.80
Caca K, Ferenci P, Kühn H et al (2001) High prevalence of the H1069Q mutation in East German patients with Wilson disease: rapid detection of mutations by limited sequencing and phenotype–genotype. Elsevier 35:575–581
Kim G-H, Kim KM, Kim J et al (2007) Identification of novel ATP7B gene mutations and their functional roles in Korean patients with Wilson disease. Hum Mutat 28:1108–1113. https://doi.org/10.1002/humu.20574
Margarit E, Bach V, Gómez D et al (2005) Mutation analysis of Wilson disease in the Spanish population - identification of a prevalent substitution and eight novel mutations in the ATP7B gene. Clin Genet 68:61–68. https://doi.org/10.1111/J.1399-0004.2005.00439.X
Penning LC, Berenguer M, Czlonkowska A et al (2023) A century of progress on Wilson disease and the enduring challenges of genetics, diagnosis, and treatment. Biomedicines 11. https://doi.org/10.3390/biomedicines11020420
Scheiber I, Brůha R, neurology PD-H of clinical (2017) Pathogenesis of Wilson disease. Elsevier 142:43–55
Gil-Bea FJ, Aldanondo G, Lasa-Fernández H et al (2017) Insights into the mechanisms of copper dyshomeostasis in amyotrophic lateral sclerosis. Expert Rev Mol Med 19:e7. https://doi.org/10.1017/erm.2017.9
Yurkova I, Arnhold J, Fitzl G et al (2011) Fragmentation of mitochondrial cardiolipin by copper ions in the Atp7b−/− mouse model of Wilson’s disease. Elsevier 164:393–400
Li M, Li Y, Chen J et al (2007) Copper ions inhibit S-adenosylhomocysteine hydrolase by causing dissociation of NAD+ cofactor. Biochemistry 46:11451–11458. https://doi.org/10.1021/BI700395D
Niculescu M, Zeisel SH (2002) Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr 132:2333S–2335S
Guo H, Zhu P, Yan L et al (2014) The DNA methylation landscape of human early embryos. nature.comH Guo, P Zhu, L Yan, R Li, B Hu, Y Lian, J Yan, X Ren, S Lin, J Li, X Jin, X Shi, P Liu, X WangNature, 2014•nature.com. 511:606–610. https://doi.org/10.1038/nature13544
Kieffer D, research VM-L (2017) Wilson disease: at the crossroads between genetics and epigenetics—a review of the evidence. Elsevier 1:121–130
Stättermayer A, Traussnigg S, Dienes H et al (2015) Hepatic steatosis in Wilson disease–Role of copper and PNPLA3 mutations. Elsevier 63:156–163
Medici V, Kieffer DA, Shibata NM et al (2016) Wilson disease: epigenetic effects of choline supplementation on phenotype and clinical course in a mouse model. Epigenetics 11:804–818. https://doi.org/10.1080/15592294.2016.1231289
Mordaunt CE, Shibata NM, Kieffer DA et al (2018) Epigenetic changes of the thioredoxin system in the tx-j mouse model and in patients with Wilson disease. Hum Mol Genet. https://doi.org/10.1093/hmg/ddy262
Burkhead JL, Gray LW, Lutsenko S (2011) Systems biology approach to Wilson’s disease. BioMetals 24:455–466. https://doi.org/10.1007/S10534-011-9430-9
Gottlieb A, Devine L, Dev S et al (2021) Steatosis development in the mouse model of Wilson disease coincides with a muted inflammatory response. In: Viszeralmedizin 2021 Gemeinsame Jahrestagung Deutsche Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS), Sektion Endoskopie der DGVS, vol 59. Deutsche Gesellschaft für Allgemein und Viszeralchirurgie (DGAV). https://doi.org/10.1055/S-0041-1733630
Hamilton JP, Koganti L, Muchenditsi A et al (2016) Activation of liver X receptor/retinoid X receptor pathway ameliorates liver disease in Atp7B−/− (Wilson disease) mice. Hepatology 63:1828–1841. https://doi.org/10.1002/HEP.28406
Huster D, Lutsenko S (2007) Wilson disease: not just a copper disorder. Analysis of a Wilson disease model demonstrates the link between copper and lipid metabolism. Mol Biosyst 3:816. https://doi.org/10.1039/b711118p
Sauer S, Merle U, Opp S et al (2011) Severe dysfunction of respiratory chain and cholesterol metabolism in Atp7b−/− mice as a model for Wilson disease. Elsevier 1812:1607–1615
Strimbu K, Tavel JA (2010) What are biomarkers? Curr Opin HIV AIDS 5:463–466. https://doi.org/10.1097/COH.0b013e32833ed177
Mohr I, Weiss KH (2019) Biochemical markers for the diagnosis and monitoring of Wilson disease. Clinical Biochemist Reviews 40:59–77. https://doi.org/10.33176/AACB-18-00014
Martins C, Costa,’ Dianne Bald Win DA, Portma B, et al (1992) Value of urinary copper excretion after penicillamine challenge in the diagnosis of Wilson’s disease. Wiley Online Library 15:609–615. https://doi.org/10.1002/hep.1840150410
Takahashi H, McCaffery J, Irizarry R et al (2006) Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol Cell 23:207–217. https://doi.org/10.1016/j.molcel.2006.05.040
Salminen A, Kauppinen A et al (2014) Krebs cycle intermediates regulate DNA and histone methylation: epigenetic impact on the aging process. Elsevier, pp 45–65
Gu M, Cooper J, Butler P et al (2000) Oxidative-phosphorylation defects in liver of patients with Wilson’s disease. The Lancet 356:469–474. https://doi.org/10.1016/S0140-6736(00)02556-3
Lekomtseva Y, Voloshyn-Gaponov I, Tatayna G (2019) Targeting higher levels of Tau protein in Ukrainian Patients with Wilson’s Disease. Neurol Ther 8:59–68. https://doi.org/10.1007/S40120-019-0134-3
Lin J, Zheng Y, Liu Y et al (2021) Higher concentration of plasma glial fibrillary acidic protein in Wilson disease patients with neurological manifestations. Movement Disord 36:1446–1450. https://doi.org/10.1002/mds.28509
Shribman S, Heller C, Burrows M (2020) Plasma neurofilament light as a biomarker of neurological involvement in Wilson’s disease. Mov Disord 36:503–508. https://doi.org/10.1002/mds.28333
Hefter H, Arslan M, Kruschel TS et al (2022) Pseudocholinesterase as a biomarker for untreated Wilson’s disease. Biomolecules 12:1791. https://doi.org/10.3390/biom12121791
Antos A, Litwin T, Przybyłkowski A et al (2022) Biomarkers of the central nervous system injury in Wilson’s disease. Pharmacotherapy in Psychiatry and Neurol 38:119–139. https://doi.org/10.5114/fpn.2022.123246
van der Ende EL, Meeter LH, Poos JM et al (2019) Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol 18:1103–1111. https://doi.org/10.1016/S1474-4422(19)30354-0
Ziemssen T, Akgun K, Członkowska A et al (2022) Serum neurofilament light chain as a biomarker of brain injury in Wilson’s disease: clinical and neuroradiological correlations. Movement Disorders 37:1074–1079. https://doi.org/10.1002/MDS.28946
Czlonkowska A, Litwin T, Karliński M et al (2014) D-penicillamine versus zinc sulfate as first-line therapy for Wilson’s disease. Eur J Neurol 21:599–606. https://doi.org/10.1111/ENE.12348
Müller J, Lichtmannegger J et al (2018) High spatial resolution LA-ICP-MS demonstrates massive liver copper depletion in Wilson disease rats upon Methanobactin treatment. Elsevier 49:119–127
Dong T, Wu M, Tang L et al (2021) GanDouLing promotes proliferation and differentiation of neural stem cells in the mouse model of Wilson’s disease. Biosci Rep 41. https://doi.org/10.1042/BSR20202717
Chen Y, Zhang B, Cao S, et al (2018) GanDouLing combined with Penicillamine improves cerebrovascular injury via PERK/eIF2α/CHOP endoplasmic reticulum stress pathway in the mouse model of. portlandpress.comY Chen, B Zhang, S Cao, W Huang, N Liu, W YangBioscience Reports, 2018•portlandpress.com 38:
Merle U, Enckea J, Tuma S et al (2006) Lentiviral gene transfer ameliorates disease progression in Long-Evans Cinnamon rats: an animal model for Wilson disease. Scand J Gastroenterol 41:974–982. https://doi.org/10.1080/00365520600554790
Leng Y, Li P, Zhou L et al (2019) Long-term correction of copper metabolism in Wilson’s disease mice with AAV8 vector delivering truncated ATP7B. Hum Gene Ther 30:1494–1504. https://doi.org/10.1089/HUM.2019.148
Wei R, Yang J, Cheng C et al (2022) CRISPR-targeted genome editing of human induced pluripotent stem cell-derived hepatocytes for the treatment of Wilson’s disease. Elsevier, p 4
Park SM, Vo K, Lallier M et al (2006) Hepatocyte transplantation in the Long Evans Cinnamon rat model of Wilson’s disease. Cell Transplant 15:13–22. https://doi.org/10.3727/000000006783982188
Sauer V, Siaj R, Stöppeler S et al (2012) Repeated transplantation of hepatocytes prevents fulminant hepatitis in a rat model of Wilson’s disease. Liver Transplant 18:248–259. https://doi.org/10.1002/lt.22466
Fujiyoshi J, Yamaza H, Sonoda S et al (2019) Therapeutic potential of hepatocyte-like-cells converted from stem cells from human exfoliated deciduous teeth in fulminant Wilson’s disease. Sci Rep 9:1535. https://doi.org/10.1038/s41598-018-38275-y
Kumar J, Sathua KB, Flora SJS (2019) Chronic copper exposure elicit neurotoxic responses in rat brain: assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and neurobehavioral parameters. Cell Mol Biol (Noisy-le-grand) 65:27–35
Patwa J, Toxicology SF-F and C (2020) MiADMSA abrogates chronic copper-induced hepatic and immunological changes in Sprague Dawley rats. Elsevier, p 45
Jedlinszki N, Kálomista I, Medicine GG-J of H (2016) Silybum marianum (milk thistle) products in Wilson’s disease: a treatment or a threat? Elsevier 6:157–159
Xu MB, Rong PQ, Jin TY et al (2019) Chinese herbal medicine for wilson’s disease: a systematic review and meta-analysis. Front Pharmacol 10:437773. https://doi.org/10.3389/FPHAR.2019.00277/BIBTEX
Lin YJ, Ho TJ, Lin TH et al (2015) P-coumaric acid regulates exon 12 splicing of the ATP7B gene by modulating hnRNP A1 protein expressions. BioMedicine (Taiwan) 5:22–30. https://doi.org/10.7603/S40681-015-0010-0
Brewer GJ (2001) Zinc acetate for the treatment of Wilson’s disease. Expert Opin Pharmacother 2:1473–1477. https://doi.org/10.1517/14656566.2.9.1473
Brewer GJ, Hedera P, Kluin KJ et al (1994) Treatment of Wilson disease with ammonium tetrathiomolybdate: III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy. Jamanetwork.com 51:545–554
Schilsky ML, Czlonkowska A, Zuin M et al (2022) Trientine tetrahydrochloride versus penicillamine for maintenance therapy in Wilson disease (CHELATE): a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol 7:1092–1102. https://doi.org/10.1016/S2468-1253(22)00270-9
Weiss KH, Askari FK, Czlonkowska A et al (2017) Bis-choline tetrathiomolybdate in patients with Wilson’s disease: an open-label, multicentre, phase 2 study. Lancet Gastroenterol Hepatol 2:869–876. https://doi.org/10.1016/S2468-1253(17)30293-5
Brewer GJ, Yuzbasiyan-Gurkan V, Johnson V et al (1993) Treatment of Wilson’s disease with zinc XII: dose regimen requirements. Am J Med Sci 305:199–202. https://doi.org/10.1097/00000441-199304000-00001
Cataldo J, Allen J, Sankoh S et al (2022) eP140: a novel, double-blind placebo-controlled seamless phase 1/2/3 AAV9 gene therapy study for Wilson disease. gimjournal.org . https://doi.org/10.1016/j.gim.2022.01.175
Ala A, Aliu E, Schilsky ML (2015) Prospective pilot study of a single daily dosage of trientine for the treatment of Wilson disease. Dig Dis Sci 60:1433–1439. https://doi.org/10.1007/S10620-014-3495-6
Study details | A controlled study of potential therapeutic effect of oral zinc in manifesting carriers of Wilson disease | ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03659331?cond=Wilson%20Disease&intr=zinc&rank=1. Accessed 27 Nov 2023
Jing Z, Daojun X, Yanbing G et al (2018) Evaluation of efficacy and safety of gandouling plus sodium dimercaptosulphonate in treatment of patients with neurological Wilson’s disease from China. Elsevier 38:781–786
Jing Z, Liangyong L, Huaizhen C et al (2018) Clinical efficacy and safety of Gandouling plus low-dose D-penicillamine for treatment of Wilson’s disease with neurological symptoms. Elsevier 38:89–94
Han Y, He G, Wang X (1999) Comparative study on therapeutic effects of gandou tablet I and dimercaptosuccinate acid in treating Wilson disease. Zhongguo Zhong Xi Yi Jie He Za Zhi 19:69–70
Gagliardi A, Giuliano E, Venkateswararao E et al (2021) Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front Pharmacol 12. https://doi.org/10.3389/FPHAR.2021.601626/FULL
Kandanapitiye MS, Wang FJ, Valley B et al (2015) Selective ion exchange governed by the irving-williams series in K2Zn3[Fe(CN)6]2 nanoparticles: toward a designer prodrug for wilsons disease. Inorg Chem 54:1212–1214. https://doi.org/10.1021/IC502957D
Patel D, Kell A, Simard B et al (2010) Cu2+-labeled, SPION loaded porous silica nanoparticles for cell labeling and multifunctional imaging probes. Elsevier 31:2866–2873
Francis C, Wroblewska L, Pegman P (2022) Systemic biodistribution and hepatocyte-specific gene editing with CRISPR/Cas9 using hyaluronic acid-based nanoparticles. Elsevier, p 40
Perera V, Liu H, Wang Z et al (2013) Cell-permeable Au@ZnMoS4 core–shell nanoparticles: toward a novel cellular copper detoxifying drug for Wilson’s disease. ACS Publications 25:4703–4709. https://doi.org/10.1021/cm402147u
Cui Z, Lockman P, Atwood C et al (2005) Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer’s and other CNS diseases. Elsevier 59:263–272
Kim SJ, Han HH, Hahn SK (2021) Hyaluronate/black phosphorus complexes as a copper chelating agent for Wilson disease treatment. Biomater Res 25. https://doi.org/10.1186/S40824-021-00221-X
Tremmel R, Uhl P, Helm F et al (2016) Delivery of copper-chelating trientine (TETA) to the central nervous system by surface modified liposomes. Elsevier 512:87–95
Gauthier L, Charbonnier P et al (2021) Development, formulation, and cellular mechanism of a lipophilic copper chelator for the treatment of Wilson’s disease. Elsevier
Acknowledgements
The authors acknowledge the Department of Pharmaceuticals under the Ministry of Chemicals and Fertilizers, Government of India. The NIPER communication number for the review article is NIPER-R/Communication number/509. Also, the authors would like to acknowledge DST-SERB (Project No. SRG/2022/001285) for their finacial support.
Funding
This work was supported by DST-SERB [SERB Project No. SRG/2022/001285].
Author information
Authors and Affiliations
Contributions
Dr. Rahul Shukla: conceptualization, review, and editing; Dr. Swapnil Sharma: formal analysis and editing; Akanksha Chaturvedi: writing, reviewing, validation, visualization.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
No animal or human studies were carried out by the authors for this article.
Consent for Publication
All authors who have been involved in completing this manuscript have given approval to the final version of the manuscript for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Wilson disease is mediated by genetic disruption in Cu(I) transporting ATPase beta polypeptide, i.e., ATP7B.
• Early diagnosis and treatment of the disease could mitigate the ailment and suppress its chronic consequences in an individual.
• Several therapies including chelation are being adapted to treat copper overload; resist at a point due to reported side effects.
• Illustration of novel therapies purporting Wilson disease.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaturvedi, A., Sharma, S. & Shukla, R. Nano-Mediated Molecular Targeting in Diagnosis and Mitigation of Wilson Disease. Mol Neurobiol (2023). https://doi.org/10.1007/s12035-023-03816-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03816-8