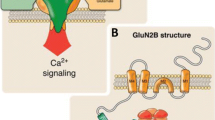
Abstract
Glycoproteins are proteins that contain oligosaccharide chains. As widely distributed functional proteins in the body, glycoproteins are essential for cellular development, cellular function maintenance, and intercellular communication. Glycoproteins not only play a role in the cell and the membrane, but they are also secreted in the intercell. These secreted glycoproteins are critical to the central nervous system for neurodevelopment and synaptic transmission. More specifically, secreted glycoproteins play indispensable roles in neurite growth mediation, axon guiding, synaptogenesis, neuronal differentiation, the release of synaptic vesicles, subunit composition of neurotransmitter receptors, and neurotransmitter receptor trafficking among other things. Abnormal expressions of secreted glycoproteins in the central nervous system are associated with abnormal neuron development, impaired synaptic organization/transmission, and neuropsychiatric disorders. This article reviews the secreted glycoproteins that regulate neuronal development and synaptic function in the central nervous system, and the molecular mechanism of these regulations, providing reference for research about synaptic function regulation and related central nervous system diseases.



Similar content being viewed by others
Data Availability
Not applicable.
References
Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H (2020) Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol 21:729–749. https://doi.org/10.1038/s41580-020-00294-x
Li C, Wang L-X (2018) Chemoenzymatic methods for the synthesis of glycoproteins. Chem Rev 118:8359–8413. https://doi.org/10.1021/acs.chemrev.8b00238
Hanover JA, Lennarz WJ (1981) Transmembrane assembly of membrane and secretory glycoproteins. Arch Biochem Biophys 211:1–19. https://doi.org/10.1016/0003-9861(81)90423-9
Messner P (1997) Bacterial glycoproteins. Glycoconj J 14:3–11. https://doi.org/10.1023/a:1018551228663
Reily C, Stewart TJ, Renfrow MB, Novak J (2019) Glycosylation in health and disease. Nat Rev Nephrol 15:346–366. https://doi.org/10.1038/s41581-019-0129-4
Bekesova S, Kosti O, Chandler KB et al (2012) N-glycans in liver-secreted and immunoglogulin-derived protein fractions. J Proteomics 75:2216–2224. https://doi.org/10.1016/j.jprot.2012.01.024
Lim DH, Kim M, Jun DW et al (2021) Diagnostic performance of serum asialo α1-acid glycoprotein levels to predict liver cirrhosis. Gut Liver 15:109–116. https://doi.org/10.5009/gnl19282
Lee Y, Bae S, Kim JH et al (2023) Diagnostic efficacy of serum asialo α1-acid glycoprotein levels for advanced liver fibrosis and cirrhosis in patients with chronic hepatitis B compared to that in healthy subjects: a prospective study. J Clin Med 12(2):712. https://doi.org/10.3390/jcm12020712
Zhang S, Shu H, Luo K et al (2011) N-linked glycan changes of serum haptoglobin β chain in liver disease patients. Mol Biosyst 7:1621–1628. https://doi.org/10.1039/c1mb05020f
Autelitano F, Loyaux D, Roudières S et al (2014) Identification of novel tumor-associated cell surface sialoglycoproteins in human glioblastoma tumors using quantitative proteomics. PLoS ONE 9:e110316. https://doi.org/10.1371/journal.pone.0110316
Zhao Z, Zhang K-N, Chai R-C et al (2019) ADAMTSL4, a secreted glycoprotein, is a novel immune-related biomarker for primary glioblastoma multiforme. Dis Markers 2019:1802620. https://doi.org/10.1155/2019/1802620
Francescone RA, Scully S, Faibish M et al (2011) Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem 286:15332–15343. https://doi.org/10.1074/jbc.M110.212514
Kim HJ, Schleiffarth JR, Jessurun J et al (2005) Wnt5 signaling in vertebrate pancreas development. BMC Biol 3:23. https://doi.org/10.1186/1741-7007-3-23
Plein A, Calmont A, Fantin A et al (2015) Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation. J Clin Invest 125:2661–2676. https://doi.org/10.1172/JCI79668
Rouyar A, Classe M, Gorski R et al (2019) Type 2/Th2-driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model. Allergy 74:549–559. https://doi.org/10.1111/all.13559
Lledo P-M, Gheusi G, Vincent J-D (2005) Information processing in the mammalian olfactory system. Physiol Rev 85:281–317. https://doi.org/10.1152/physrev.00008.2004
Ambrogioni L, Ólafsdóttir HF (2023) Rethinking the hippocampal cognitive map as a meta-learning computational module. Trends Cogn Sci 27:702–712. https://doi.org/10.1016/j.tics.2023.05.011
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353:777–783. https://doi.org/10.1126/science.aag2590
Guo L, Tian J, Du H (2017) Mitochondrial dysfunction and synaptic transmission failure in Alzheimer’s disease. J Alzheimers Dis 57:1071–1086. https://doi.org/10.3233/JAD-160702
Nusse R, Clevers H (2017) Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169:985–999. https://doi.org/10.1016/j.cell.2017.05.016
Li D, Sun J, Zhong TP (2022) Wnt signaling in heart development and regeneration. Curr Cardiol Rep 24:1425–1438. https://doi.org/10.1007/s11886-022-01756-8
Steinhart Z, Angers S (2018) Wnt signaling in development and tissue homeostasis. Development 145(11):dev146589. https://doi.org/10.1242/dev.146589
Swarup S, Verheyen EM (2012) Wnt/wingless signaling in Drosophila. Cold Spring Harb Perspect Biol 4(6):a007930. https://doi.org/10.1101/cshperspect.a007930
Moon RT (2005) Wnt/beta-catenin pathway. Sci STKE. 271 cm1. https://doi.org/10.1126/stke.2712005cm1
Serafino A, Giovannini D, Rossi S, Cozzolino M (2020) Targeting the Wnt/β-catenin pathway in neurodegenerative diseases: recent approaches and current challenges. Expert Opin Drug Discov 15:803–822. https://doi.org/10.1080/17460441.2020.1746266
Willert K, Nusse R (2012) Wnt proteins. Cold Spring Harb Perspect Biol 4:a007864. https://doi.org/10.1101/cshperspect.a007864
Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5:367–377. https://doi.org/10.1016/s1534-5807(03)00266-1
Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10:468–477. https://doi.org/10.1038/nrm2717
Gordon MD, Nusse R (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281:22429–22433. https://doi.org/10.1074/jbc.R600015200
Kohn AD, Moon RT (2005) Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium 38:439–446. https://doi.org/10.1016/j.ceca.2005.06.022
Lukaszewicz AI, Anderson DJ (2011) Cyclin D1 promotes neurogenesis in the developing spinal cord in a cell cycle-independent manner. Proc Natl Acad Sci USA 108:11632–11637. https://doi.org/10.1073/pnas.1106230108
Kuwabara T, Hsieh J, Muotri A et al (2009) Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci 12:1097–1105. https://doi.org/10.1038/nn.2360
Miranda CJ, Braun L, Jiang Y et al (2012) Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell 11:542–552. https://doi.org/10.1111/j.1474-9726.2012.00816.x
Ingraham CA, Park GC, Makarenkova HP, Crossin KL (2011) Matrix metalloproteinase (MMP)-9 induced by Wnt signaling increases the proliferation and migration of embryonic neural stem cells at low O2 levels. J Biol Chem 286:17649–57. https://doi.org/10.1074/jbc.M111.229427
Hirabayashi Y, Itoh Y, Tabata H et al (2004) The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131:2791–2801. https://doi.org/10.1242/dev.01165
Inestrosa NC, Montecinos-Oliva C, Fuenzalida M (2012) Wnt signaling: role in Alzheimer disease and schizophrenia. J Neuroimmune Pharmacol 7:788–807. https://doi.org/10.1007/s11481-012-9417-5
Wang Q, Huang X, Su Y et al (2022) Activation of Wnt/β-catenin pathway mitigates blood-brain barrier dysfunction in Alzheimer’s disease. Brain 145:4474–4488. https://doi.org/10.1093/brain/awac236
Joksimovic M, Awatramani R (2014) Wnt/β-catenin signaling in midbrain dopaminergic neuron specification and neurogenesis. J Mol Cell Biol 6:27–33. https://doi.org/10.1093/jmcb/mjt043
Chen C-M, Orefice LL, Chiu S-L et al (2017) Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. Proc Natl Acad Sci USA 114:E619–E628. https://doi.org/10.1073/pnas.1615792114
Arredondo SB, Guerrero FG, Herrera-Soto A et al (2020) Wnt5a promotes differentiation and development of adult-born neurons in the hippocampus by noncanonical Wnt signaling. Stem Cells 38:422–436. https://doi.org/10.1002/stem.3121
Cerpa W, Gambrill A, Inestrosa NC, Barria A (2011) Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J Neurosci 31:9466–71. https://doi.org/10.1523/JNEUROSCI.6311-10.2011
Varela-Nallar L, Alfaro IE, Serrano FG et al (2010) Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci USA 107:21164–21169. https://doi.org/10.1073/pnas.1010011107
Cerpa W, Farías GG, Godoy JA et al (2010) Wnt-5a occludes Abeta oligomer-induced depression of glutamatergic transmission in hippocampal neurons. Mol Neurodegener 5:3. https://doi.org/10.1186/1750-1326-5-3
Muñoz FJ, Godoy JA, Cerpa W et al (2014) Wnt-5a increases NO and modulates NMDA receptor in rat hippocampal neurons. Biochem Biophys Res Commun 444:189–194. https://doi.org/10.1016/j.bbrc.2014.01.031
Cuitino L, Godoy JA, Farías GG et al (2010) Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J Neurosci 30:8411–8420. https://doi.org/10.1523/JNEUROSCI.5736-09.2010
Farías GG, Alfaro IE, Cerpa W et al (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 284:15857–15866. https://doi.org/10.1074/jbc.M808986200
Wallace J, Lutgen V, Avasarala S et al (2018) Wnt7a induces a unique phenotype of monocyte-derived macrophages with lower phagocytic capacity and differential expression of pro- and anti-inflammatory cytokines. Immunology 153:203–213. https://doi.org/10.1111/imm.12830
Ciani L, Boyle KA, Dickins E et al (2011) Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca2+/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci USA 108:10732–10737. https://doi.org/10.1073/pnas.1018132108
Cerpa W, Godoy JA, Alfaro I et al (2008) Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem 283:5918–5927. https://doi.org/10.1074/jbc.M705943200
Wayman GA, Impey S, Marks D et al (2006) Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron 50:897–909. https://doi.org/10.1016/j.neuron.2006.05.008
Stein E, Tessier-Lavigne M (2001) Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291:1928–1938. https://doi.org/10.1126/science.1058445
Prasad A, Fernandis AZ, Rao Y, Ganju RK (2004) Slit protein-mediated inhibition of CXCR4-induced chemotactic and chemoinvasive signaling pathways in breast cancer cells. J Biol Chem 279:9115–9124. https://doi.org/10.1074/jbc.M308083200
Marlow R, Strickland P, Lee JS et al (2008) SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4 within breast epithelium. Cancer Res 68:7819–7827. https://doi.org/10.1158/0008-5472.CAN-08-1357
Nguyen-Ba-Charvet KT, Chédotal A (2002) Role of Slit proteins in the vertebrate brain. J Physiol Paris 96:91–98. https://doi.org/10.1016/s0928-4257(01)00084-5
Nguyen-Ba-Charvet KT, Plump AS, Tessier-Lavigne M, Chedotal A (2002) Slit1 and slit2 proteins control the development of the lateral olfactory tract. J Neurosci 22:5473–80. https://doi.org/10.1523/JNEUROSCI.22-13-05473.2002
Miyasaka N, Sato Y, Yeo S-Y et al (2005) Robo2 is required for establishment of a precise glomerular map in the zebrafish olfactory system. Development 132:1283–1293. https://doi.org/10.1242/dev.01698
Plump AS, Erskine L, Sabatier C et al (2002) Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron 33:219–232. https://doi.org/10.1016/s0896-6273(01)00586-4
Bagri A, Marín O, Plump AS et al (2002) Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33:233–248. https://doi.org/10.1016/s0896-6273(02)00561-5
Li Y, Gao Y, Xu X et al (2017) Slit2/Robo1 promotes synaptogenesis and functional recovery of spinal cord injury. NeuroReport 28:75–81. https://doi.org/10.1097/WNR.0000000000000715
Bai B, Wang X, Li Y et al (2020) Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 105:975-991.e7. https://doi.org/10.1016/j.neuron.2019.12.015
Li J, Han L, Wen Y et al (2015) Increased permeability of the blood-brain barrier and Alzheimer’s disease-like alterations in slit-2 transgenic mice. J Alzheimers Dis 43:535–548. https://doi.org/10.3233/JAD-141215
Miura E, Iijima T, Yuzaki M, Watanabe M (2006) Distinct expression of Cbln family mRNAs in developing and adult mouse brains. Eur J Neurosci 24:750–60. https://doi.org/10.1111/j.1460-9568.2006.04950.x
Südhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455:903–911. https://doi.org/10.1038/nature07456
Craig AM, Kang Y (2007) Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol 17:43–52. https://doi.org/10.1016/j.conb.2007.01.011
Matsuda K, Yuzaki M (2011) Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur J Neurosci 33:1447–1461. https://doi.org/10.1111/j.1460-9568.2011.07638.x
Matsuda K, Miura E, Miyazaki T et al (2010) Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science 328:363–368. https://doi.org/10.1126/science.1185152
Wei P, Pattarini R, Rong Y et al (2012) The Cbln family of proteins interact with multiple signaling pathways. J Neurochem 121:717–729. https://doi.org/10.1111/j.1471-4159.2012.07648.x
Iijima T, Miura E, Matsuda K et al (2007) Characterization of a transneuronal cytokine family Cbln–regulation of secretion by heteromeric assembly. Eur J Neurosci 25:1049–1057. https://doi.org/10.1111/j.1460-9568.2007.05361.x
Hirai H, Pang Z, Bao D et al (2005) Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci 8:1534–1541. https://doi.org/10.1038/nn1576
Seigneur E, Wang J, Dai J et al (2021) Cerebellin-2 regulates a serotonergic dorsal raphe circuit that controls compulsive behaviors. Mol Psychiatry 26:7509–7521. https://doi.org/10.1038/s41380-021-01187-x
Sterky FH, Trotter JH, Lee S-J et al (2017) Carbonic anhydrase-related protein CA10 is an evolutionarily conserved pan-neurexin ligand. Proc Natl Acad Sci USA 114:E1253–E1262. https://doi.org/10.1073/pnas.1621321114
Barembaum M, Moreno TA, LaBonne C et al (2000) Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nat Cell Biol 2:219–225. https://doi.org/10.1038/35008643
Nakaya N, Sultana A, Lee H-S, Tomarev SI (2012) Olfactomedin 1 interacts with the Nogo A receptor complex to regulate axon growth. J Biol Chem 287:37171–84. https://doi.org/10.1074/jbc.M112.389916
Rice HC, Townsend M, Bai J et al (2012) Pancortins interact with amyloid precursor protein and modulate cortical cell migration. Development 139:3986–3996. https://doi.org/10.1242/dev.082909
Moreno TA, Bronner-Fraser M (2005) Noelins modulate the timing of neuronal differentiation during development. Dev Biol 288:434–447. https://doi.org/10.1016/j.ydbio.2005.09.050
Nakaya N, Sultana A, Munasinghe J et al (2013) Deletion in the N-terminal half of olfactomedin 1 modifies its interaction with synaptic proteins and causes brain dystrophy and abnormal behavior in mice. Exp Neurol 250:205–218. https://doi.org/10.1016/j.expneurol.2013.09.019
Nakaya N, Sultana A, Tomarev SI (2017) Impaired AMPA receptor trafficking by a double knockout of zebrafish olfactomedin1a/b. J Neurochem 143:635–644. https://doi.org/10.1111/jnc.14231
Pandya NJ, Seeger C, Babai N et al (2018) Noelin1 affects lateral mobility of synaptic AMPA receptors. Cell Rep 24:1218–1230. https://doi.org/10.1016/j.celrep.2018.06.102
AyraultJarrier M, Levy G, Polonovski J (1963) Study of humanserum alpha-lipoproteins by immunoelectrophoresis. Bull Soc Chim Biol (Paris) 45:703–13
Flower DR (1996) The lipocalin protein family: structure and function. Biochem J 318(Pt 1):1–14. https://doi.org/10.1042/bj3180001
Yin J, Spillman E, Cheng ES et al (2021) Brain-specific lipoprotein receptors interact with astrocyte derived apolipoprotein and mediate neuron-glia lipid shuttling. Nat Commun 12:2408. https://doi.org/10.1038/s41467-021-22751-7
Kalman J, McConathy W, Araoz C et al (2000) Apolipoprotein D in the aging brain and in Alzheimer’s dementia. Neurol Res 22:330–336. https://doi.org/10.1080/01616412.2000.11740678
Belloir B, Kövari E, Surini-Demiri M, Savioz A (2001) Altered apolipoprotein D expression in the brain of patients with Alzheimer disease. J Neurosci Res 64:61–9. https://doi.org/10.1002/jnr.1054
Martínez E, Navarro A, Ordóñez C et al (2012) Amyloid-β25-35 induces apolipoprotein D synthesis and growth arrest in HT22 hippocampal cells. J Alzheimers Dis 30:233–244. https://doi.org/10.3233/JAD-2012-112102
Li H, Ruberu K, Muñoz SS et al (2015) Apolipoprotein D modulates amyloid pathology in APP/PS1 Alzheimer’s disease mice. Neurobiol Aging 36:1820–1833. https://doi.org/10.1016/j.neurobiolaging.2015.02.010
Hanayama R, Tanaka M, Miwa K et al (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417:182–187. https://doi.org/10.1038/417182a
Luo Y, Lu D, Zhou J et al (2023) Milk fat globule epidermal growth factor 8a regulates neurogenesis in telencephalon and affects larval behavior in zebrafish. Stem Cells Dev 32:246–257. https://doi.org/10.1089/scd.2022.0247
Kurematsu C, Sawada M, Ohmuraya M et al (2022) Synaptic pruning of murine adult-born neurons by microglia depends on phosphatidylserine. J Exp Med 219(4):e20202304. https://doi.org/10.1084/jem.20202304
Funding
This study was funded by the Graduate Student Scientific Research Innovation Projects in Jiangsu Province (grant No. KYCX22_1928).
Author information
Authors and Affiliations
Contributions
This review was designed by Sen Liu and Haiying Gong. Haiying Gong conceived the article and revised the manuscript. Liu Sen analyzed the data, wrote the manuscript, and revised the revision. Conglei Zhu and Di Han modified the pictures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have given final approval of the version and agreed with the publication of this study here.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, H., Zhu, C., Han, D. et al. Secreted Glycoproteins That Regulate Synaptic Function: the Dispatchers in the Central Nervous System. Mol Neurobiol 61, 2719–2727 (2024). https://doi.org/10.1007/s12035-023-03731-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03731-y