Abstract
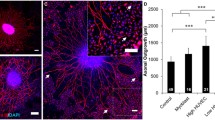
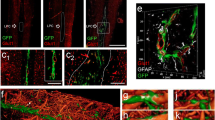
The vascular and the nervous systems share similarities in addition to their complex role in providing oxygen and nutrients to all cells. Both are highly branched networks that frequently grow close to one another during development. Vascular patterning and neural wiring share families of guidance cues and receptors. Most recently, this relationship has been investigated in terms of peripheral nervous system (PNS) regeneration, where nerves and blood vessels often run in parallel so endothelial cells guide the formation of the Büngner bands which support axonal regeneration. Here, we characterized the vascular response in regenerative models of the central and peripheral nervous system. After sciatic nerve crush, followed by axon regeneration, there was a significant increase in the blood vessel density 7 days after injury. In addition, the optic nerve crush model was used to evaluate intrinsic regenerative potential activated with a combined treatment that stimulated retinal ganglion cells (RGCs) regrowth. We observed that a 2-fold change in the total number of blood vessels occurred 7 days after optic nerve crush compared to the uncrushed nerve. The difference increased up to a 2.7-fold change 2 weeks after the crush. Interestingly, we did not observe differences in the total number of blood vessels 2 weeks after crush, compared to animals that had received combined treatment for regeneration and controls. Therefore, the vascular characterization showed that the increase in vascular density was not related to the efficiency of both peripheral and central axonal regeneration.




Similar content being viewed by others
Data Availability
The data supporting the findings of this study are available in Github at https://github.com/victorresend/Barbara-s-manuscript-files.git, reference number: #001. These data were derived from the following resources available in the public domain: (https://drive.google.com/drive/folders/164kwm_07smXNLJEUnofZVPwhTX6lO7j?usp=share_link and https://drive.google.com/drive/folders/1-Gi36nlNth%2D%2DntbHnxHPU52BN332-zK4?usp=share_link).
References
Wälchli T, Bisschop J, Carmeliet P, Zadeh G, Monnier PP, De Bock K, Radovanovic I (2023) Shaping the brain vasculature in development and disease in the single-cell era. Nat Rev Neurosci 24(5):271–298. https://doi.org/10.1038/s41583-023-00684-y
Sapieha P (2012) Eyeing central neurons in vascular growth and reparative angiogenesis. Blood 120(11):2182–2194. https://doi.org/10.1182/blood-2012-04-396846
Wakayama Y, Yamagishi S (2023) Vascular and neuronal network formation regulated by growth factors and guidance cues. Life (Basel) 13(2). https://doi.org/10.3390/life13020283
Zochodne DW (2018) Local blood flow in peripheral nerves and their ganglia: resurrecting key ideas around its measurement and significance. Muscle Nerve 57(6):884–895. https://doi.org/10.1002/mus.26031
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436(7048):193–200. https://doi.org/10.1038/nature03875
Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ (2002) Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109(6):693–705. https://doi.org/10.1016/s0092-8674(02)00757-2
Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC (2015) Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 162(5):1127–1139. https://doi.org/10.1016/j.cell.2015.07.021
Vargas ME, Barres BA (2007) Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci 30:153–179. https://doi.org/10.1146/annurev.neuro.30.051606.094354
Oh WJ, Gu C (2013) Establishment of neurovascular congruency in the mouse whisker system by an independent patterning mechanism. Neuron 80(2):458–469. https://doi.org/10.1016/j.neuron.2013.09.005
Erskine L, François U, Denti L, Joyce A, Tillo M, Bruce F, Vargesson N, Ruhrberg C (2017) VEGF-A and neuropilin 1 (NRP1) shape axon projections in the developing CNS via dual roles in neurons and blood vessels. Development 144(13):2504–2516. https://doi.org/10.1242/dev.151621
Guest JD, Hiester ED, Bunge RP (2005) Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol 192(2):384–393. https://doi.org/10.1016/j.expneurol.2004.11.033
Garcia-Diaz B, Bachelin C, Coulpier F, Gerschenfeld G, Deboux C, Zujovic V, Charnay P, Topilko P, Baron-Van Evercooren A (2019) Blood vessels guide Schwann cell migration in the adult demyelinated CNS through Eph/ephrin signaling. Acta Neuropathol 138(3):457–476. https://doi.org/10.1007/s00401-019-02011-1
Saffari TM, Bedar M, Hundepool CA, Bishop AT, Shin AY (2020) The role of vascularization in nerve regeneration of nerve graft. Neural Regen Res 15(9):1573–1579. https://doi.org/10.4103/1673-5374.276327
de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L (2012) Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci USA 109(23):9149–9154. https://doi.org/10.1073/pnas.1119449109
Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD (2016) Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci 19(8):1073–1084. https://doi.org/10.1038/nn.4340
Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z (2011) Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480(7377):372–375. https://doi.org/10.1038/nature10594
Curcio M, Bradke F (2018) Axon regeneration in the central nervous system: facing the challenges from the inside. Annu Rev Cell Dev Biol 34:495–521. https://doi.org/10.1146/annurev-cellbio-100617-062508
Iadecola C (2017) The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96(1):17–42. https://doi.org/10.1016/j.neuron.2017.07.030
Caillaud M, Richard L, Vallat JM, Desmoulière A, Billet F (2019) Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res 14(1):24–33. https://doi.org/10.4103/1673-5374.243699
Podhajsky RJ, Myers RR (1993) The vascular response to nerve crush: relationship to Wallerian degeneration and regeneration. Brain Res 623(1):117–123. https://doi.org/10.1016/0006-8993(93)90018-i
Pola R, Aprahamian TR, Bosch-Marcé M, Curry C, Gaetani E, Flex A, Smith RC, Isner JM, Losordo DW (2004) Age-dependent VEGF expression and intraneural neovascularization during regeneration of peripheral nerves. Neurobiol Aging 25(10):1361–1368. https://doi.org/10.1016/j.neurobiolaging.2004.02.028
Ribeiro-Resende VT, Pimentel-Coelho PM, Mesentier-Louro LA, Mendez RM, Mello-Silva JP, Cabral-da-Silva MC, de Mello FG, de Melo Reis RA, Mendez-Otero R (2009) Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience 159(2):540–549. https://doi.org/10.1016/j.neuroscience.2008.12.059
Ribeiro-Resende VT, Oliveira-Silva A, Ouverney-Brandão S, Santiago MF, Hedin-Pereira C, Mendez-Otero R (2007) Ganglioside 9-O-acetyl GD3 expression is upregulated in the regenerating peripheral nerve. Neuroscience 147(1):97–105. https://doi.org/10.1016/j.neuroscience.2007.03.046
Nakajima Y, Sakabe M, Matsui H, Sakata H, Yanagawa N, Yamagishi T (2009) Heart development before beating. Anat Sci Int 84(3):67–76. https://doi.org/10.1007/s12565-009-0025-2
Sun Y, Smith LEH (2018) Retinal Vasculature in development and diseases. Annu Rev Vis Sci 4:101–122. https://doi.org/10.1146/annurev-vision-091517-034018
Chang CP, Bruneau BG (2012) Epigenetics and cardiovascular development. Annu Rev Physiol 74:41–68. https://doi.org/10.1146/annurev-physiol-020911-153242
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322(5903):963–966. https://doi.org/10.1126/science.1161566
Benowitz LI, He Z, Goldberg JL (2017) Reaching the brain: advances in optic nerve regeneration. Exp Neurol 287(Pt 3):365–373. https://doi.org/10.1016/j.expneurol.2015.12.015
Morrison JC, Johnson EC, Cepurna WO, Funk RH (1999) Microvasculature of the rat optic nerve head. Invest Ophthalmol Vis Sci 40(8):1702–1709
May CA, Lütjen-Drecoll E (2002) Morphology of the murine optic nerve. Invest Ophthalmol Vis Sci 43(7):2206–2212
Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274(5290):1123–1133. https://doi.org/10.1126/science.274.5290.1123
McLaughlin T, O'Leary DD (2005) Molecular gradients and development of retinotopic maps. Annu Rev Neurosci 28:327–355. https://doi.org/10.1146/annurev.neuro.28.061604.135714
Rigby MJ, Gomez TM, Puglielli L (2020) Glial cell-axonal growth cone interactions in neurodevelopment and regeneration. Front Neurosci 14:203. https://doi.org/10.3389/fnins.2020.00203
Suter T, Jaworski A (2019) Cell migration and axon guidance at the border between central and peripheral nervous system. Science 365(6456). https://doi.org/10.1126/science.aaw8231
Sloan SA, Barres BA (2014) Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol 27:75–81. https://doi.org/10.1016/j.conb.2014.03.005
Dravid A, Parittotokkaporn S, Aqrawe Z, O'Carroll SJ, Svirskis D (2020) Determining neurotrophin gradients in vitro to direct axonal outgrowth following spinal cord injury. ACS Chem Nerosci 11(2):121–132. https://doi.org/10.1021/acschemneuro.9b00565
Ebadi M, Bashir RM, Heidrick ML, Hamada FM, Refaey HE, Hamed A, Helal G, Baxi MD, Cerutis DR, Lassi NK (1997) Neurotrophins and their receptors in nerve injury and repair. Neurochem Int 30(4-5):347–374. https://doi.org/10.1016/s0197-0186(96)00071-x
Evaristo-Mendonça F, Sardella-Silva G, Kasai-Brunswick TH, Campos RMP, Domizi P, Santiago MF, de Melo Reis RA, Mendez-Otero R, Ribeiro-Resende VT, Pimentel-Coelho PM (2019) Preconditioning of rat bone marrow-derived mesenchymal stromal cells with toll-like receptor agonists. Stem Cells International 2019:1–18. https://doi.org/10.1155/2019/7692973
Conforti L, Gilley J, Coleman MP (2014) Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci 15(6):394–409. https://doi.org/10.1038/nrn3680
Park KK, Liu K, Hu Y, Kanter JL, He Z (2010) PTEN/mTOR and axon regeneration. Exp Neurol 223(1):45–50. https://doi.org/10.1016/j.expneurol.2009.12.032
Eichmann A, Thomas JL (2013) Molecular parallels between neural and vascular development. Cold Spring Harb Perspect Med 3(1):a006551. https://doi.org/10.1101/cshperspect.a006551
Zarkada G, Howard JP, Xiao X, Park H, Bizou M, Leclerc S, Künzel SE, Boisseau B, Li J, Cagnone G, Joyal JS, Andelfinger G, Eichmann A, Dubrac A (2021) Specialized endothelial tip cells guide neuroretina vascularization and blood-retina-barrier formation. Dev Cell 56(15):2237–2251.e2236. https://doi.org/10.1016/j.devcel.2021.06.021
Cross MJ, Claesson-Welsh L (2001) FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 22(4):201–207. https://doi.org/10.1016/s0165-6147(00)01676-x
Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Bréant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A (2004) The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 432(7014):179–186. https://doi.org/10.1038/nature03080
Larrivée B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L, Bouvrée K, Bréant C, Del Toro R, Bréchot N, Germain S, Bono F, Dol F, Claes F, Fischer C, Autiero M, Thomas JL, Carmeliet P, Tessier-Lavigne M, Eichmann A (2007) Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev 21(19):2433–2447. https://doi.org/10.1101/gad.437807
Koch AW, Mathivet T, Larrivée B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvrée K, Stawicki S, Nicholes K, Rathore N, Scales SJ, Luis E, del Toro R, Freitas C, Bréant C, Michaud A, Corvol P, Thomas J-L, Wu Y et al (2011) Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell 20(1):33–46. https://doi.org/10.1016/j.devcel.2010.12.001
Brunet I, Gordon E, Han J, Cristofaro B, Broqueres-You D, Liu C, Bouvrée K, Zhang J, del Toro R, Mathivet T, Larrivée B, Jagu J, Pibouin-Fragner L, Pardanaud L, Machado MJ, Kennedy TE, Zhuang Z, Simons M, Levy BI et al (2014) Netrin-1 controls sympathetic arterial innervation. J Clin Invest 124(7):3230–3240. https://doi.org/10.1172/jci75181
Aalkjær C, Nilsson H, De Mey JGR (2021) Sympathetic and sensory-motor nerves in peripheral small arteries. Physiol Rev 101(2):495–544. https://doi.org/10.1152/physrev.00007.2020
Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G (2000) Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected]. J Biol Chem 275(24):18040–18045. https://doi.org/10.1074/jbc.M909259199
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. https://doi.org/10.1146/annurev.neuro.24.1.677
Alsina FC, Irala D, Fontanet PA, Hita FJ, Ledda F, Paratcha G (2012) Sprouty4 is an endogenous negative modulator of TrkA signaling and neuronal differentiation induced by NGF. PloS One 7(2):e32087. https://doi.org/10.1371/journal.pone.0032087
Gonzalez A, Moya-Alvarado G, Gonzalez-Billaut C, Bronfman FC (2016) Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton (Hoboken) 73(10):612–628. https://doi.org/10.1002/cm.21312
Gysler SM, Drapkin R (2021) Tumor innervation: peripheral nerves take control of the tumor microenvironment. J Clin Invest 131(11). https://doi.org/10.1172/jci147276
Acknowledgements
The authors are grateful to Luciano Cavalcante for the laboratory technical support. We also are grateful for grant support from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro, and the National Institute for Translational Neuroscience.
Funding
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq VTRR 2014; CNPq VTRR 2018, CNPq RAMR PQ2), the Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (Faperj VTRR 2013; Faperj VTRR 2015; Faperj RAMR 2019), and the INCT-INNT (RAMR National Institute for Translational Neuroscience).
Author information
Authors and Affiliations
Contributions
BRS: performed all the animal handling experiments, tissue processing, immunolabeling, and quantitative analysis; analyzed the data imaging by optical microscopy; and wrote and discussed the manuscript. RAMR: analyzed the data and wrote and discussed the manuscript. VTRR: general coordinator, analyzed the data, and discussed and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All Animal handling and surgical procedures were carried out in accordance with the approved guidelines for the Use of Animals in Research from the Federal University of Rio de Janeiro (CEUA IBCCF protocol#175-18) and from National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, B.R., de Melo Reis, R.A. & Ribeiro-Resende, V.T. A Comparative Investigation of Axon-Blood Vessel Growth Interaction in the Regenerating Sciatic and Optic Nerves in Adult Mice. Mol Neurobiol 61, 2215–2227 (2024). https://doi.org/10.1007/s12035-023-03705-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03705-0