Abstract
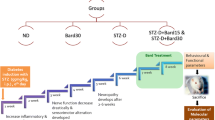
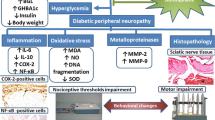
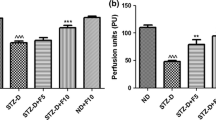
Diabetic painful neuropathy (DPN) is one of the most detrimental complications of diabetes. Alterations in neuroinflammatory mediators play significant roles in the development of DPN. Infiltration of the neutrophils and monocyte/macrophages contributes substantial role in the degenerative process of the distal sciatic nerve by forming neutrophil extracellular traps (NETs) under diabetic condition. Citrullination of histones due to increase in protein arginine deiminase (PAD) enzyme activity under hyperglycemia may promote NET formation, which can further increase the cytokine production by activating macrophages and proliferation of neutrophils. This study reveals that the increase in histone deacetylases (HDAC) is crucial in DPN and inhibition of HDAC using HDAC inhibitor (HDACi) FK228 would suppress NETosis and alleviate diabetic nerve degeneration and pain. FK228, also known as romidepsin, is FDA approved for the treatment of cutaneous T-cell lymphoma yet the molecular mechanisms of this drug are not completely understood in DPN. In this study, type 2 diabetic (T2D) mice with pain were treated with HDACi, FK228 1 mg/kg; I.P. 2 × /week for 3 weeks. The results demonstrate that FK228 treatment can alleviate thermal hyperalgesia and mechanical allodynia significantly along with changes in the expression of HDACs in the dorsal root ganglia (DRG) and spinal cord dorsal horn neurons of diabetic animals. The results also indicate that FK228 treatment can alter the expression of neutrophil elastase (NE), extracellular or cell free DNA (cfDNA), citrullinated histone-3 (CitH3), PADI4, growth-associated protein (GAP)-43, and glucose transporter (GLUT)-4. Overall, this study suggests that FK228 could amend the expression of nerve regeneration markers and inflammatory mediators in diabetic animals and may offer an alternative treatment approach for DPN.









Similar content being viewed by others
Data Availability
All data generated or analyzed during the current study are included in this published article. Additional data will be available from the corresponding author on reasonable request.
Abbreviations
- DRG:
-
Dorsal root ganglia
- HDAC:
-
Histone deacetylase
- T2D:
-
Type 2 diabetes
- TLR4:
-
Toll-like receptor 4
- H3K9ac:
-
Histone 3 lysine (K) 9 acetylation
- FK228:
-
Romidepsin
- NE:
-
Neutrophil elastase
- cfDNA:
-
Cell-free DNA
- CitH3:
-
Citrullinated histone 3
- GAP43:
-
Growth-associated protein 43
- PAD:
-
Peptidyl arginine deiminase
- NET:
-
Neutrophil extracellular trap
- GLUT4:
-
Glucose transporter type 4
References
Spallone V (2012) Management of painful diabetic neuropathy: guideline guidance or jungle? Curr Diab Rep 12:403
Javed S et al (2015) Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis 6(1):15–28
Lindsay TJ et al (2010) Treating diabetic peripheral neuropathic pain. Am Fam Physician 82(2):151–158
Azmi S et al (2019) Pregabalin in the management of painful diabetic neuropathy: a narrative review. Diabetes Ther 10(1):35–56
Derry S et al (2017) Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev 1:007393
Agathos E et al (2018) Effect of alpha-lipoic acid on symptoms and quality of life in patients with painful diabetic neuropathy. J Int Med Res 46(5):1779–1790
Fraser DA et al (2012) The effects of long-term oral benfotiamine supplementation on peripheral nerve function and inflammatory markers in patients with type 1 diabetes: a 24-month, double-blind, randomized, placebo-controlled trial. Diabetes Care 35(5):1095–1097
Yang J, Yaron A, Liu K (2022) Editorial: Neuroimmune interactions in peripheral neuropathy. Front Mol Neurosci 15:929081
Shouman K, Benarroch EE (2021) Peripheral neuroimmune interactions: selected review and some clinical implications. Clin Auton Res 31(4):477–489
Meade E, Garvey M (2022) The role of neuro-immune interaction in chronic pain conditions; functional somatic syndrome, neurogenic inflammation, and peripheral neuropathy. Int J Mol Sci 23(15):8574
Niemi JP, Lindborg JA, Zigmond RE (2020) Detection of neutrophils in the sciatic nerve following peripheral nerve injury. Methods Mol Biol 2143:207–222
Wong SL et al (2015) Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 21(7):815–819
Wang L et al (2018) Hyperglycemia induces neutrophil extracellular traps formation through an NADPH oxidase-dependent pathway in diabetic retinopathy. Front Immunol 9:3076
Wang Y et al (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184(2):205–213
Thalin C et al (2018) Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One 13(1):e0191231
Shafqat A et al (2022) Emerging role of neutrophil extracellular traps in the complications of diabetes mellitus. Front Med (Lausanne) 9:995993
Gaub P et al (2011) The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain 134(Pt 7):2134–2148
Matsushita Y et al (2013) HDAC inhibitors restore C-fibre sensitivity in experimental neuropathic pain model. Br J Pharmacol 170(5):991–998
Gaub P et al (2010) HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ 17(9):1392–1408
Basu T et al (2019) Histone deacetylase inhibitors restore normal hippocampal synaptic plasticity and seizure threshold in a mouse model of tuberous sclerosis complex. Sci Rep 9(1):5266
Ganai SA, Ramadoss M, Mahadevan V (2016) Histone deacetylase (HDAC) inhibitors - emerging roles in neuronal memory, learning, synaptic plasticity and neural regeneration. Curr Neuropharmacol 14(1):55–71
Chen YT et al (2012) Expression patterns of histone deacetylases in experimental stroke and potential targets for neuroprotection. Clin Exp Pharmacol Physiol 39(9):751–758
Denk F et al (2013) HDAC inhibitors attenuate the development of hypersensitivity in models of neuropathic pain. Pain 154(9):1668–1679
Chung D, Shum A, Caraveo G (2020) GAP-43 and BASP1 in axon regeneration: implications for the treatment of neurodegenerative diseases. Front Cell Dev Biol 8:567537
Fantini F, Johansson O (1992) Expression of growth-associated protein 43 and nerve growth factor receptor in human skin: a comparative immunohistochemical investigation. J Invest Dermatol 99(6):734–742
Bursova S et al (2012) Expression of growth-associated protein 43 in the skin nerve fibers of patients with type 2 diabetes mellitus. J Neurol Sci 315(1–2):60–63
McNay EC, Pearson-Leary J (2020) GluT4: a central player in hippocampal memory and brain insulin resistance. Exp Neurol 323:113076
Ashrafi G et al (2017) GLUT4 mobilization supports energetic demands of active synapses. Neuron 93(3):606-615 e3
Passarelli M, Machado UFF (2021) AGEs-induced and endoplasmic reticulum stress/inflammation-mediated regulation of GLUT4 expression and atherogenesis in diabetes mellitus. Cells 11(1):104
Langer HT et al (2020) Nerve damage induced skeletal muscle atrophy is associated with increased accumulation of intramuscular glucose and polyol pathway intermediates. Sci Rep 10(1):1908
Molteni M, Gemma S, Rossetti C (2016) The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediators Inflamm 2016:6978936
Halili MA et al (2010) Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the toll-like receptor 4 agonist LPS. J Leukoc Biol 87(6):1103–1114
Hamam HJ, Palaniyar N (2019) Histone deacetylase inhibitors dose-dependently switch neutrophil death from NETosis to apoptosis. Biomolecules 9(5):184
Frye R et al (2012) Romidepsin: a new drug for the treatment of cutaneous T-cell lymphoma. Clin J Oncol Nurs 16(2):195–204
Kleinman AJ et al (2020) Pharmacokinetics and immunological effects of romidepsin in rhesus macaques. Front Immunol 11:579158
Sturm D et al (2014) Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer 14(2):92–107
Hargreaves K et al (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32(1):77–88
Chaplan SR et al (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Chattopadhyay M, Mata M, Fink DJ (2008) Continuous delta-opioid receptor activation reduces neuronal voltage-gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. J Neurosci 28(26):6652–8
Verdu E et al (1999) Physiological and immunohistochemical characterization of cisplatin-induced neuropathy in mice. Muscle Nerve 22(3):329–340
Benbow SJ, Wallymahmed ME, MacFarlane IA (1998) Diabetic peripheral neuropathy and quality of life. QJM 91(11):733–737
Ogurtsova K et al (2017) IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50
Nathan DM (1993) Long-term complications of diabetes mellitus. N Engl J Med 328(23):1676–1685
Kanbayashi H et al (2002) Spatial distribution of nociceptive neuropeptide and nerve growth factor depletion in experimental diabetic peripheral nervous system. J Int Med Res 30(5):512–519
Dewanjee S et al (2021) The emerging role of HDACs: pathology and therapeutic targets in diabetes mellitus. Cells 10(6):1340
Cho Y, Cavalli V (2014) HDAC signaling in neuronal development and axon regeneration. Curr Opin Neurobiol 27:118–126
Wang Z, Zhao YT, Zhao TC (2021) Histone deacetylases in modulating cardiac disease and their clinical translational and therapeutic implications. Exp Biol Med (Maywood) 246(2):213–225
Zhu D, Zhang Y, Wang S (2021) Histone citrullination: a new target for tumors. Mol Cancer 20(1):90
Baumann D et al (2021) p38 MAPK signaling in M1 macrophages results in selective elimination of M2 macrophages by MEK inhibition. J Immunother Cancer 9(7):e002319
Wang G, et al (2015) HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A 112(9):2853-2858
Thakur V, Sadanandan J, Chattopadhyay M (2020) High-mobility group box 1 protein signaling in painful diabetic neuropathy. Int J Mol Sci 21(3):881
Sun Q et al (2019) Downregulation of glucose-6-phosphate dehydrogenase contributes to diabetic neuropathic pain through upregulation of toll-like receptor 4 in rats. Mol Pain 15:1744806919838659
Moser VA, Uchoa MF, Pike CJ (2018) TLR4 inhibitor TAK-242 attenuates the adverse neural effects of diet-induced obesity. J Neuroinflammation 15(1):306
Agthong S, Tomlinson DR (2002) Inhibition of p38 MAP kinase corrects biochemical and neurological deficits in experimental diabetic neuropathy. Ann N Y Acad Sci 973:359–362
Du Y et al (2010) Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci 51(4):2158–2164
Mohiuddin L, Tomlinson DR (1997) Impaired molecular regenerative responses in sensory neurones of diabetic rats: gene expression changes in dorsal root ganglia after sciatic nerve crush. Diabetes 46(12):2057–2062
Weems JC, Griesel BA, Olson AL (2012) Class II histone deacetylases downregulate GLUT4 transcription in response to increased cAMP signaling in cultured adipocytes and fasting mice. Diabetes 61(6):1404–1414
Acknowledgements
We thank our Laboratory Animal Resources Center (LARC) staffs for taking care of the animals.
Funding
This work was supported by the start-up fund for Munmun Chattopadhyay from Texas Tech University Health Sciences Center, El Paso, TX.
Author information
Authors and Affiliations
Contributions
Vikram Thakur (VT) and Munmun Chattopadhyay (MC) designed the study. VT, Mayra Gonzalez conducted the animal experiments and collected data. Maria Parada and Robert Martinez performed tissue analysis and in vitro studies. All authors were involved in analysis and interpretation of the data, drafting and finalizing the manuscript. MC supervised the study and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were performed in compliance with approved institutional animal care and use protocols (IACUC, TTUHSC El Paso, TX) in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved facility. All animal experiments followed the instructions from “Guide for the Care and Use of Laboratory Animals” 8th edition, 2011.
Consent for Publication
Not applicable.
Consent to Participate
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1. Increased expression of HDAC2 in hyperglycemic DRG neuronal cells ameliorated by FK228 treatment
High glucose exposure (25mM) for 48 hours increased the expression of HDAC2 significantly in F11 DRG cell line when compared with normoglycemic condition. Overnight treatment with FK228 lowered the HDAC2 expression in hyperglycemic cells that were exposed to high glucose 24 hours prior to treatment. (EPS 5306 KB)
12035_2023_3701_MOESM2_ESM.eps
Supplementary file2. Increased H3K9 acetylation was observed in hyperglycemic DRG neuronal cell line treated with HDAC inhibitor
Immunocytochemical studies revealed that treatment with HDAC inhibitor FK228 increased H3K9 acetylation in treated hyperglycemic DRG neurons compared to hyperglycemic and normoglycemic DRG neuronal cell line. Although not significant, hyperglycemic DRG cells showed a downregulation of H3K9 acetylation compared to normoglycemic DRG neuronal cells. (EPS 12639 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thakur, V., Gonzalez, M.A., Parada, M. et al. Role of Histone Deacetylase Inhibitor in Diabetic Painful Neuropathy. Mol Neurobiol 61, 2283–2296 (2024). https://doi.org/10.1007/s12035-023-03701-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03701-4