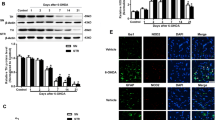
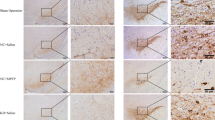
Abstract
Both neuroinflammation and iron accumulation play roles in the pathogenesis of Parkinson’s disease (PD). However, whether inflammation induces iron dyshomeostasis in dopaminergic neurons at an early stage of PD, at which no quantifiable dopaminergic neuron loss can be observed, is still unknown. As for the inflammation mediators, although several cytokines have been reported to increase in PD, the functions of these cytokines in the SN are double-edged and controversial. In this study, whether inflammation could induce iron dyshomeostasis in dopaminergic neurons through high mobility group protein B1 (HMGB1) in the early stage of PD is explored. Lipopolysaccharide (LPS), a toxin that primarily activates glia cells, and 6-hydroxydopamine (6-OHDA), the neurotoxin that firstly impacts dopaminergic neurons, were utilized to mimic PD in rats. We found a common and exceedingly early over-production of HMGB1, followed by an increase of divalent metal transporter 1 with iron responsive element (DMT1+) in the dopaminergic neurons before quantifiable neuronal loss. HMGB1 neutralizing antibody suppressed inflammation in the SN, DMT1+ elevation in dopaminergic neurons, and dopaminergic neuronal loss in both LPS and 6-OHDA administration- induced PD models. On the contrary, interleukin-1β inhibitor diacerein failed to suppress these outcomes induced by 6-OHDA. Our findings not only demonstrate that inflammation could be one of the causes of DMT1+ increase in dopaminergic neurons, but also highlight HMGB1 as a pivotal early mediator of inflammation-induced iron increase and subsequent neurodegeneration, thereby HMGB1 could serve as a potential target for early-stage PD treatment.






Similar content being viewed by others
Data Availability
Further information and requests for data and materials will be shared upon receipt of a reasonable request.
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- DMT1+:
-
Divalent metal transporter 1 with iron responsive element
- ED-1:
-
Anti-macrophages/monocytes antibody, clone ED-1
- GFAAS:
-
Graphite furnace atomic absorption spectroscopy
- GFAP:
-
Glial fibrillary acidic protein
- HMGB1:
-
High mobility group protein B1
- IBA1:
-
Ionized calcium-binding adaptor molecule 1
- IL-1β:
-
Interleukin-1β
- LPS:
-
Lipopolysaccharide
- PD:
-
Parkinson’s disease
- SN:
-
Substantia nigra
- RAGE:
-
Receptor for advanced glycation end products
- TNF-α:
-
Tumor necrosis factor-α
References
Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG et al (2019) Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurol 18(5):459–480. https://doi.org/10.1016/s1474-4422(18)30499-x
Mochizuki H, Choong CJ, Baba K (2020) Parkinson’s disease and iron. J Neural Transmis (Vienna, Austria : 1996) 127(2):181–187. https://doi.org/10.1007/s00702-020-02149-3
Jiang H, Song N, Jiao Q, Shi L, Du X (2019) Iron pathophysiology in Parkinson diseases. Adv Exp Med Biol 1173:45–66. https://doi.org/10.1007/978-981-13-9589-5_4
Ndayisaba A, Kaindlstorfer C, Wenning GK (2019) Iron in neurodegeneration — cause or consequence? Front Neurosci 13:180. https://doi.org/10.3389/fnins.2019.00180
Bergsland N, Tavazzi E, Schweser F, Jakimovski D, Hagemeier J, Dwyer MG, Zivadinov R (2019) Targeting iron dyshomeostasis for treatment of neurodegenerative disorders. CNS Drugs 33(11):1073–1086. https://doi.org/10.1007/s40263-019-00668-6
Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R et al (2003) Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron 37(6):899–909. https://doi.org/10.1016/S0896-6273(03)00126-0
Shachar DB, Kahana N, Kampel V, Warshawsky A, Youdim MB (2004) Neuroprotection by a novel brain permeable iron chelator, VK-28, against 6-hydroxydopamine lession in rats. Neuropharmacology 46(2):254–263. https://doi.org/10.1016/j.neuropharm.2003.09.005
Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS (2019) Emerging therapies in Parkinson disease — repurposed drugs and new approaches. Nat Rev Neurol 15(4):204–223. https://doi.org/10.1038/s41582-019-0155-7
Crichton RR, Ward RJ, Hider RC (2019) The efficacy of iron chelators for removing iron from specific brain regions and the pituitary-ironing out the brain. Pharmaceuticals (Basel, Switzerland) 12(3). https://doi.org/10.3390/ph12030138
Nuñez MT, Chana-Cuevas P (2018) New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals (Basel, Switzerland) 11(4). https://doi.org/10.3390/ph11040109
Liang T, Qian ZM, Mu MD, Yung WH, Ke Y (2020) Brain hepcidin suppresses major pathologies in experimental Parkinsonism. iScience 23(7):101284. https://doi.org/10.1016/j.isci.2020.101284
Ke Y, Qian ZM (2007) Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol 83(3):149–173. https://doi.org/10.1016/j.pneurobio.2007.07.009
Jiang H, Song N, Xu H, Zhang S, Wang J, Xie J (2010) Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res 20(3):345–356. https://doi.org/10.1038/cr.2010.20
Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C et al (2008) Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci U S A 105(47):18578–18583. https://doi.org/10.1073/pnas.0804373105
Urrutia PJ, Aguirre P, Tapia V, Carrasco CM, Mena NP, Núñez MT (2017) Cell death induced by mitochondrial complex I inhibition is mediated by iron regulatory protein 1. Biochim Biophys Acta Mol basis Dis 1863(9):2202–2209. https://doi.org/10.1016/j.bbadis.2017.05.015
Song N, Jiang H, Wang J, Xie JX (2007) Divalent metal transporter 1 up-regulation is involved in the 6-hydroxydopamine-induced ferrous iron influx. J Neurosci Res 85(14):3118–3126. https://doi.org/10.1002/jnr.21430
Chen D, Kanthasamy AG, Reddy MB (2015) EGCG protects against 6-OHDA-induced neurotoxicity in a cell culture model. Parkinson’s Dis 2015:843906. https://doi.org/10.1155/2015/843906
Pajares M, Ir A, Manda G, Boscá L, Cuadrado A (2020) Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells 9(7). https://doi.org/10.3390/cells9071687
Deleidi M, Gasser T (2013) The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol Life Sci: CMLS 70(22):4259–4273. https://doi.org/10.1007/s00018-013-1352-y
Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, Dexter DT (2009) Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J Neurochem 110(3):966–975. https://doi.org/10.1111/j.1471-4159.2009.06189.x
Lazdon E, Stolero N, Frenkel D (2020) Microglia and Parkinson’s disease: footprints to pathology. J Neural Transmis 127(2):149–158. https://doi.org/10.1007/s00702-020-02154-6
Thomsen MS, Andersen MV, Christoffersen PR, Jensen MD, Lichota J, Moos T (2015) Neurodegeneration with inflammation is accompanied by accumulation of iron and ferritin in microglia and neurons. Neurobiol Dis 81:108–118. https://doi.org/10.1016/j.nbd.2015.03.013
Zhang J, Stanton DM, Nguyen XV, Liu M, Zhang Z, Gash D, Bing G (2005) Intrapallidal lipopolysaccharide injection increases iron and ferritin levels in glia of the rat substantia nigra and induces locomotor deficits. Neuroscience 135(3):829–838. https://doi.org/10.1016/j.neuroscience.2005.06.049
Zhang Z, Hou L, Song JL, Song N, Sun YJ, Lin X, Wang XL, Zhang FZ et al (2014) Pro-inflammatory cytokine-mediated ferroportin down-regulation contributes to the nigral iron accumulation in lipopolysaccharide-induced Parkinsonian models. Neuroscience 257:20–30. https://doi.org/10.1016/j.neuroscience.2013.09.037
Zhang Z, Zhang K, Du X, Li Y (2012) Neuroprotection of desferrioxamine in lipopolysaccharide-induced nigrostriatal dopamine neuron degeneration. Mol Med Rep 5(2):562–566. https://doi.org/10.3892/mmr.2011.671
Wang J, Song N, Jiang H, Wang J, Xie J (2013) Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim Biophys Acta 1832(5):618–625. https://doi.org/10.1016/j.bbadis.2013.01.021
Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, Gonzalez-Billault C, Nunez MT (2013) Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 126(4):541–549. https://doi.org/10.1111/jnc.12244
Qian ZM, He X, Liang T, Wu KC, Yan YC, Lu LN, Yang G, Luo QQ et al (2014) Lipopolysaccharides upregulate hepcidin in neuron via microglia and the IL-6/STAT3 signaling pathway. Mol Neurobiol 50(3):811–820. https://doi.org/10.1007/s12035-014-8671-3
Mu MD, Qian ZM, Yang SX, Rong KL, Yung WH, Ke Y (2020) Therapeutic effect of a histone demethylase inhibitor in Parkinson’s disease. Cell Death Dis 11(10):927. https://doi.org/10.1038/s41419-020-03105-5
Subbarayan MS, Hudson C, Moss LD, Nash KR, Bickford PC (2020) T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an α-synuclein rat model of Parkinson’s disease. J Neuroinflammation 17(1):242. https://doi.org/10.1186/s12974-020-01911-4
Li Q, Ke Y, Chan Danny CW, Qian Z-M, Yung Ken KL, Ko H, Arbuthnott Gordon W, Yung W-H (2012) Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 76(5):1030–1041. https://doi.org/10.1016/j.neuron.2012.09.032
Li C-J, Zhang L-G, Liu L-B, An M-Q, Dong L-g GH-Y, Dai Y-P, Wang F, Mao C-J et al (2022) Inhibition of spinal 5-HT3 receptor and spinal dorsal horn neuronal excitability alleviates hyperalgesia in a rat model of Parkinson’s disease. Mol Neurobiol 59(12):7253–7264. https://doi.org/10.1007/s12035-022-03034-8
Rumpel R, Hohmann M, Klein A, Wesemann M, Baumgärtner W, Ratzka A, Grothe C (2015) Transplantation of fetal ventral mesencephalic progenitor cells overexpressing high molecular weight fibroblast growth factor 2 isoforms in 6-hydroxydopamine lesioned rats. Neuroscience 286:293–307. https://doi.org/10.1016/j.neuroscience.2014.11.060
Huotarinen A, Leino S, Tuominen RK, Laakso A (2019) Rat subthalamic stimulation: evaluating stimulation-induced dyskinesias, choosing stimulation currents and evaluating the anti-akinetic effect in the cylinder test. MethodsX 6:2384–2395. https://doi.org/10.1016/j.mex.2019.10.012
Magno LAV, Collodetti M, Tenza-Ferrer H, Romano-Silva MA (2019) Cylinder test to assess sensory-motor function in a mouse model of Parkinson’s disease. Bio-protocol 9(16):e3337. https://doi.org/10.21769/BioProtoc.3337
Almezgagi M, Zhang Y, Hezam K, Shamsan E, Gamah M, Al-Shaebi F, Abbas AB, Shoaib M et al (2020) Diacerein: recent insight into pharmacological activities and molecular pathways. Biomed Pharmacot 131:110594. https://doi.org/10.1016/j.biopha.2020.110594
Baizabal-Carvallo JF, Alonso-Juarez M (2020) The link between gut dysbiosis and neuroinflammation in Parkinson’s disease. Neuroscience 432:160–173. https://doi.org/10.1016/j.neuroscience.2020.02.030
Jackson A, Forsyth CB, Shaikh M, Voigt RM, Engen PA, Ramirez V, Keshavarzian A (2019) Diet in Parkinson’s disease: critical role for the microbiome. Front Neurol 10:1245. https://doi.org/10.3389/fneur.2019.01245
Dutta G, Zhang P, Liu B (2008) The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol 22(5):453–464. https://doi.org/10.1111/j.1472-8206.2008.00616.x
Brown GC (2019) The endotoxin hypothesis of neurodegeneration. J Neuroinflammation 16(1):180. https://doi.org/10.1186/s12974-019-1564-7
Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A 100(14):8514–8519. https://doi.org/10.1073/pnas.1432609100
Milanese C, Tapias V, Gabriels S, Cerri S, Levandis G, Blandini F, Tresini M, Shiva S et al (2018) Mitochondrial complex I reversible S-nitrosation improves bioenergetics and is protective in Parkinson’s disease. Antioxid Redox Signal 28(1):44–61. https://doi.org/10.1089/ars.2017.6992
Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM (1995) Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience 67(3):631–647. https://doi.org/10.1016/0306-4522(95)00066-r
Puspita L, Chung SY, Shim JW (2017) Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain 10(1):53. https://doi.org/10.1186/s13041-017-0340-9
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3(4):461–491. https://doi.org/10.3233/jpd-130230
Jellinger K, Linert L, Kienzl E, Herlinger E, Youdim MB (1995) Chemical evidence for 6-hydroxydopamine to be an endogenous toxic factor in the pathogenesis of Parkinson’s disease. J Neural Transm Suppl 46:297–314
Borie C, Gasparini F, Verpillat P, Bonnet AM, Agid Y, Hetet G, Brice A, Durr A et al (2002) Association study between iron-related genes polymorphisms and Parkinson’s disease. J Neurol 249(7):801–804. https://doi.org/10.1007/s00415-002-0704-6
He Q, Du T, Yu X, Xie A, Song N, Kang Q, Yu J, Tan L et al (2011) DMT1 polymorphism and risk of Parkinson’s disease. Neurosci Lett 501(3):128–131. https://doi.org/10.1016/j.neulet.2011.07.001
Rhodes SL, Buchanan DD, Ahmed I, Taylor KD, Loriot MA, Sinsheimer JS, Bronstein JM, Elbaz A et al (2014) Pooled analysis of iron-related genes in Parkinson’s disease: association with transferrin. Neurobiol Dis 62:172–178. https://doi.org/10.1016/j.nbd.2013.09.019
Funke C, Schneider SA, Berg D, Kell DB (2013) Genetics and iron in the systems biology of Parkinson’s disease and some related disorders. Neurochem Int 62(5):637–652. https://doi.org/10.1016/j.neuint.2012.11.015
Muñoz Y, Carrasco CM, Campos JD, Aguirre P, Núñez MT (2016) Parkinson’s disease: the mitochondria-iron link. Parkinson’s disease 2016:7049108. https://doi.org/10.1155/2016/7049108
Liddell JR, White AR (2018) Nexus between mitochondrial function, iron, copper and glutathione in Parkinson’s disease. Neurochem Int 117:126–138. https://doi.org/10.1016/j.neuint.2017.05.016
Cerri S, Milanese C, Mastroberardino PG (2019) Endocytic iron trafficking and mitochondria in Parkinson’s disease. Int J Biochem Cell Biol 110:70–74. https://doi.org/10.1016/j.biocel.2019.02.009
Mena NP, Urrutia PJ, Lourido F, Carrasco CM, Núñez MT (2015) Mitochondrial iron homeostasis and its dysfunctions in neurodegenerative disorders. Mitochondrion 21:92–105. https://doi.org/10.1016/j.mito.2015.02.001
Mena NP, Bulteau AL, Salazar J, Hirsch EC, Núñez MT (2011) Effect of mitochondrial complex I inhibition on Fe–S cluster protein activity. Biochem Biophys Res Commun 409(2):241–246. https://doi.org/10.1016/j.bbrc.2011.04.137
Teismann P, Sathe K, Bierhaus A, Leng L, Martin HL, Bucala R, Weigle B, Nawroth PP et al (2012) Receptor for advanced glycation endproducts (RAGE) deficiency protects against MPTP toxicity. Neurobiol Aging 33(10):2478–2490. https://doi.org/10.1016/j.neurobiolaging.2011.12.006
Gasparotto J, Ribeiro CT, Bortolin RC, Somensi N, Rabelo TK, Kunzler A, Souza NC, Pasquali MAB et al (2017) Targeted inhibition of RAGE in substantia nigra of rats blocks 6-OHDA-induced dopaminergic denervation. Sci Rep 7(1):8795. https://doi.org/10.1038/s41598-017-09257-3
Jiang X, Wang X, Tuo M, Ma J, Xie A (2018) RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci Lett 672:65–69. https://doi.org/10.1016/j.neulet.2018.02.049
Li Y, Qin M, Zhong W, Liu C, Deng G, Yang M, Li J, Ye H et al (2023) RAGE promotes dysregulation of iron and lipid metabolism in alcoholic liver disease. Redox Biol 59:102559. https://doi.org/10.1016/j.redox.2022.102559
Gan P, Ding L, Hang G, Xia Q, Huang Z, Ye X, Qian X (2020) Oxymatrine attenuates dopaminergic neuronal damage and microglia-mediated neuroinflammation through cathepsin D-dependent HMGB1/TLR4/NF-κB pathway in Parkinson’s disease. Front Pharmacol 11:776. https://doi.org/10.3389/fphar.2020.00776
Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS (2011) HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31(3):1081–1092. https://doi.org/10.1523/jneurosci.3732-10.2011
Mitra S, Chakrabarti N, Bhattacharyya A (2011) Differential regional expression patterns of alpha-synuclein, TNF-alpha, and IL-1beta; and variable status of dopaminergic neurotoxicity in mouse brain after Paraquat treatment. J Neuroinflammation 8:163. https://doi.org/10.1186/1742-2094-8-163
Koprich JB, Reske-Nielsen C, Mithal P, Isacson O (2008) Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation 5:8. https://doi.org/10.1186/1742-2094-5-8
Jiang Y, Ma H, Wang X, Wang Z, Yang Y, Li L, Feng T (2020) Protective effect of the α7 nicotinic receptor agonist PNU-282987 on dopaminergic neurons against 6-hydroxydopamine, regulating anti-neuroinflammatory and the immune balance pathways in rat. Front Aging Neurosci 12:606927. https://doi.org/10.3389/fnagi.2020.606927
Tiefensee Ribeiro C, Peixoto DO, Santos L, Saibro-Girardi C, Brum PO, Carazza-Kessler FG, Somensi N, Behrens LMP et al (2021) Intranasal HSP70 administration protects against dopaminergic denervation and modulates neuroinflammatory response in the 6-OHDA rat model. Brain Behav Immun health 14:100253. https://doi.org/10.1016/j.bbih.2021.100253
Stojakovic A, Paz-Filho G, Arcos-Burgos M, Licinio J, Wong ML, Mastronardi CA (2017) Role of the IL-1 pathway in dopaminergic neurodegeneration and decreased voluntary movement. Mol Neurobiol 54(6):4486–4495. https://doi.org/10.1007/s12035-016-9988-x
Castaño A, Herrera AJ, Cano J, Machado A (2002) The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-alpha, IL-1beta and IFN-gamma. J Neurochem 81(1):150–157. https://doi.org/10.1046/j.1471-4159.2002.00799.x
Depino AM, Earl C, Kaczmarczyk E, Ferrari C, Besedovsky H, del Rey A, Pitossi FJ, Oertel WH (2003) Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson’s disease. Eur J Neurosci 18(10):2731–2742. https://doi.org/10.1111/j.1460-9568.2003.03014.x
Niu H, Wang Q, Zhao W, Liu J, Wang D, Muhammad B, Liu X, Quan N et al (2020) IL-1β/IL-1R1 signaling induced by intranasal lipopolysaccharide infusion regulates alpha-synuclein pathology in the olfactory bulb, substantia nigra and striatum. Brain Pathol(Zurich, Switzerland). https://doi.org/10.1111/bpa.12886
Pott Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ (2008) Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain J Neurol 131(Pt 7):1880–1894. https://doi.org/10.1093/brain/awn101
Yepuri G, Shekhtman A, Marie Schmidt A, Ramasamy R (2021) Heme & RAGE: a new opportunistic relationship? FEBS J 288(11):3424–3427. https://doi.org/10.1111/febs.15723
Liu X, Nemeth DP, McKim DB, Zhu L, DiSabato DJ, Berdysz O, Gorantla G, Oliver B et al (2019) Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity 50(2):317–333.e316. https://doi.org/10.1016/j.immuni.2018.12.012
An Y, Chen Q, Quan N (2011) Interleukin-1 exerts distinct actions on different cell types of the brain in vitro. J Inflamm Res 2011(4):11–20. https://doi.org/10.2147/jir.s15357
Walsh S, Gavin A, Wyatt S, O'Connor C, Keeshan K, Nolan YM, O'Keeffe GW, Sullivan AM (2015) Knockdown of interleukin-1 receptor 1 is not neuroprotective in the 6-hydroxydopamine striatal lesion rat model of Parkinson's disease. Int J Neurosci 125(1):70–77. https://doi.org/10.3109/00207454.2014.904304
Yi PL, Tsai CH, Lu MK, Liu HJ, Chen YC, Chang FC (2007) Interleukin-1beta mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep 30(4):413–425. https://doi.org/10.1093/sleep/30.4.413
Joppe K, Roser AE, Maass F, Lingor P (2019) The contribution of iron to protein aggregation disorders in the central nervous system. Front Neurosci 13:15. https://doi.org/10.3389/fnins.2019.00015
Ganguly U, Banerjee A, Chakrabarti SS, Kaur U, Sen O, Cappai R, Chakrabarti S (2020) Interaction of α-synuclein and Parkin in iron toxicity on SH-SY5Y cells: implications in the pathogenesis of Parkinson's disease. Biochem J 477(6):1109–1122. https://doi.org/10.1042/bcj20190676
Esteves AR, Silva DF, Banha D, Candeias E, Guedes B, Cardoso SM (2023) LPS-induced mitochondrial dysfunction regulates innate immunity activation and α-synuclein oligomerization in Parkinson's disease. Redox Biol 63:102714. https://doi.org/10.1016/j.redox.2023.102714
Zhang FX, Xu RS (2018) Juglanin ameliorates LPS-induced neuroinflammation in animal models of Parkinson's disease and cell culture via inactivating TLR4/NF-κB pathway. Biomed Pharmacother 97:1011–1019. https://doi.org/10.1016/j.biopha.2017.08.132
Deng I, Corrigan F, Zhai G, Zhou XF, Bobrovskaya L (2020) Lipopolysaccharide animal models of Parkinson's disease: Recent progress and relevance to clinical disease. Brain Behav Immun Health 4:100060. https://doi.org/10.1016/j.bbih.2020.100060
Zheng Q, Ma P, Yang P, Zhai S, He M, Zhang X, Tu Q, Jiao L et al (2023) Alpha lipoic acid ameliorates motor deficits by inhibiting ferroptosis in Parkinson's disease. Neurosci Lett 810:137346. https://doi.org/10.1016/j.neulet.2023.137346
Song LM, Xiao ZX, Zhang N, Yu XQ, Cui W, Xie JX, Xu HM (2021) Apoferritin improves motor deficits in MPTP-treated mice by regulating brain iron metabolism and ferroptosis. iScience 24(5):102431. https://doi.org/10.1016/j.isci.2021.102431
Sun J, Lin XM, Lu DH, Wang M, Li K, Li SR, Li ZQ, Zhu CJ et al (2023) Midbrain dopamine oxidation links ubiquitination of glutathione peroxidase 4 to ferroptosis of dopaminergic neurons. J Clin Invest 133(10). https://doi.org/10.1172/jci165228
Funding
This work was supported by the Hong Kong Research Grants Council-General Research Fund, 14115821, 14113522, Collaborative Research Fund, C4012-22GF, Health and Medical Research Fund, 7180906.
Author information
Authors and Affiliations
Contributions
Y.K. conceived the project, designed the experiments, and supervised the project. W.H.Y. and Z.M.Q. designed the experiments, supervised the studies, and analyzed the data. T.L., S.X.Y., and L.D.D. designed the experiments, performed the experiments, analyzed the data, and prepared the figures. Y.K., C.Q., and T.L. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The Animal Ethics and Experimentation Committee of the Chinese University of Hong Kong approved the use of animals for this study (Ref No. 14-068-GRF), and all experiments follow the relevant regulatory standards.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, T., Yang, SX., Qian, C. et al. HMGB1 Mediates Inflammation-Induced DMT1 Increase and Dopaminergic Neurodegeneration in the Early Stage of Parkinsonism. Mol Neurobiol 61, 2006–2020 (2024). https://doi.org/10.1007/s12035-023-03668-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03668-2