Abstract
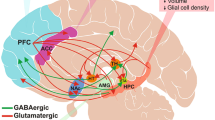
Postpartum depression is a serious disease with a high incidence and severe impact on pregnant women and infants, but its mechanism remains unclear. Recent studies have shown that GABA receptors, especially extrasynaptic receptors, are closely associated with postpartum depression. There are many different structures of GABA receptors, so different types of receptors have different functions, even though they transmit information primarily through GABA. In this review, we focus on the function of GABA receptors, especially extrasynaptic GABA receptors, and their association with postpartum depression. We have shown that the extrasynaptic GABA receptor has a significant impact on the activity and function of neurons through tonic inhibition. The extrasynaptic receptor and its ligands undergo drastic changes during pregnancy and childbirth. Abnormal changes or the body’s inability to adjust and recover may be an important cause of postpartum depression. Finally, by reviewing the mechanisms of several novel antidepressants, we suggest that extrasynaptic receptors may be potential targets for the treatment of postpartum depression.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author.
References
Wang Z, Liu J, Shuai H, Cai Z, Fu X, Liu Y, Xiao X, Zhang W et al (2021) Mapping global prevalence of depression among postpartum women. Transl Psychiatry 11(1):543. https://doi.org/10.1038/s41398-021-01663-6
O'Hara MW, McCabe JE (2013) Postpartum depression: current status and future directions. Annu Rev Clin Psychol 9:379–407. https://doi.org/10.1146/annurev-clinpsy-050212-185612
Leung BM, Kaplan BJ (2009) Perinatal depression: prevalence, risks, and the nutrition link--a review of the literature. J Am Diet Assoc 109(9):1566–1575. https://doi.org/10.1016/j.jada.2009.06.368
Lee YL, Tien Y, Bai YS, Lin CK, Yin CS, Chung CH, Sun CA, Huang SH et al (2022) Association of postpartum depression with maternal suicide: a nationwide population-based study. Int J Environ Res Public Health 19(9):5118. https://doi.org/10.3390/ijerph19095118
Bauer AE, Liu X, Byrne EM, Sullivan PF, Wray NR, Agerbo E, Nyegaard M, Grove J et al (2019) Genetic risk scores for major psychiatric disorders and the risk of postpartum psychiatric disorders. Transl Psychiatry 9(1):288. https://doi.org/10.1038/s41398-019-0629-9
Mahon PB, Payne JL, MacKinnon DF, Mondimore FM, Goes FS, Schweizer B, Jancic D, NIMH Genetics Initiative Bipolar Disorder Consortium; BiGS Consortium et al (2009) Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry 166(11):1229–1237. https://doi.org/10.1176/appi.ajp.2009.09030417
Zhao XH, Zhang ZH (2020) Risk factors for postpartum depression: an evidence-based systematic review of systematic reviews and meta-analyses. Asian J Psychiatr 53:102353. https://doi.org/10.1016/j.ajp.2020.102353
Gastaldon C, Solmi M, Correll CU, Barbui C, Schoretsanitis G (2022) Risk factors of postpartum depression and depressive symptoms: umbrella review of current evidence from systematic reviews and meta-analyses of observational studies. Br J Psychiatry 221(4):591–602. https://doi.org/10.1192/bjp.2021.222
Kim JH, Kim JY, Lee S, Lee S, Stubbs B, Koyanagi A, Dragioti E, Jacob L et al (2022) Environmental risk factors, protective factors, and biomarkers for postpartum depressive symptoms: an umbrella review. Neurosci Biobehav Rev 140:104761. https://doi.org/10.1016/j.neubiorev.2022.104761
Wang SY, Duan KM, Tan XF, Yin JY, Mao XY, Zheng W, Wang CY, Yang M et al (2017) Genetic variants of the kynurenine-3-monooxygenase and postpartum depressive symptoms after cesarean section in Chinese women. J Affect Disord 215:94–101. https://doi.org/10.1016/j.jad.2017.03.023
McEvoy K, Osborne LM, Nanavati J, Payne JL (2017) Reproductive affective disorders: a review of the genetic evidence for premenstrual dysphoric disorder and postpartum depression. Curr Psychiatry Rep 19(12):94
Page CE, Coutellier L (2019) Prefrontal excitatory/inhibitory balance in stress and emotional disorders: evidence for over-inhibition. Neurosci Biobehav Rev 105:39–51. https://doi.org/10.1016/j.neubiorev.2019.07.024
Fogaça MV, Duman RS (2019) Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci 12(13):87. https://doi.org/10.3389/fncel.2019.00087
Marchisella F, Creutzberg KC, Begni V, Sanson A, Wearick-Silva LE, Tractenberg SG, Orso R, Kestering-Ferreira É et al (2021) Exposure to prenatal stress is associated with an excitatory/inhibitory imbalance in rat prefrontal cortex and amygdala and an increased risk for emotional dysregulation. Front Cell Dev Biol 1(9):653384. https://doi.org/10.3389/fcell.2021.653384
Gaiarsa JL, Porcher C (2013) Emerging neurotrophic role of GABAB receptors in neuronal circuit development. Front Cell Neurosci 12(7):206. https://doi.org/10.3389/fncel.2013.00206
Sigel E, Steinmann ME (2012) Structure, function, and modulation of GABA(A) receptors. J Biol Chem 287(48):40224–40231. https://doi.org/10.1074/jbc.R112.386664
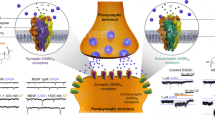
Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6(3):215–229. https://doi.org/10.1038/nrn1625
Kasaragod VB, Mortensen M, Hardwick SW, Wahid AA, Dorovykh V, Chirgadze DY, Smart TG, Miller PS (2022) Mechanisms of inhibition and activation of extrasynaptic αβ GABAA receptors. Nature 602(7897):529–533. https://doi.org/10.1038/s41586-022-04402-z
Hannan S, Minere M, Harris J, Izquierdo P, Thomas P, Tench B, Smart TG (2020) GABAAR isoform and subunit structural motifs determine synaptic and extrasynaptic receptor localisation. Neuropharmacol 1(169):107540. https://doi.org/10.1016/j.neuropharm.2019.02.022
Mostallino MC, Sanna E, Concas A, Biggio G, Follesa P (2009) Plasticity and function of extrasynaptic GABA(A) receptors during pregnancy and after delivery. Psychoneuroendocrinol 34(Suppl 1):S74–S83. https://doi.org/10.1016/j.psyneuen.2009.06.013
Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439(7076):589–593. https://doi.org/10.1038/nature04404
Mazzone GL, Nistri A (2019) Modulation of extrasynaptic GABAergic receptor activity influences glutamate release and neuronal survival following excitotoxic damage to mouse spinal cord neurons. Neurochem Int 128:175–185. https://doi.org/10.1016/j.neuint.2019.04.018
Davenport CM, Rajappa R, Katchan L, Taylor CR, Tsai MC, Smith CM, de Jong JW, Arnold DB et al (2021) Relocation of an extrasynaptic GABAA receptor to inhibitory synapses freezes excitatory synaptic strength and preserves memory. Neuron 109(1):123–134. https://doi.org/10.1016/j.neuron.2020.09.037
Mody I, Pearce RA (2004) Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci 27(9):569–575. https://doi.org/10.1016/j.tins.2004.07.002
Semyanov A, Walker MC, Kullmann DM (2003) GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6(5):484–490. https://doi.org/10.1038/nn1043
Lee V, Maguire J (2014) The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits 3(8):3. https://doi.org/10.3389/fncir.2014.00003
Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR (2002) GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol 446(2):179–197. https://doi.org/10.1002/cne.10210
Ferando I, Mody I (2013) Altered gamma oscillations during pregnancy through loss of δ subunit-containing GABA(A) receptors on parvalbumin interneurons. Front Neural Circuits 17(7):144. https://doi.org/10.3389/fncir.2013.00144
Milenkovic I, Vasiljevic M, Maurer D, Höger H, Klausberger T, Sieghart W (2013) The parvalbumin-positive interneurons in the mouse dentate gyrus express GABAA receptor subunits α1, β2, and δ along their extrasynaptic cell membrane. Neurosci 19(254):80–96. https://doi.org/10.1016/j.neuroscience.2013.09.019
Udakis M, Pedrosa V, Chamberlain SEL, Clopath C, Mellor JR (2020) Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat Commun 11(1):4395. https://doi.org/10.1038/s41467-020-18074-8.
Drasbek KR, Jensen K (2006) THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex 16(8):1134–1141. https://doi.org/10.1093/cercor/bhj055
Maguire J (2019) Neuroactive steroids and GABAergic involvement in the neuroendocrine dysfunction associated with major depressive disorder and postpartum depression. Front Cell Neurosci 8(13):83. https://doi.org/10.3389/fncel.2019.00083
Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H (2004) Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 24(6):1478–1485. https://doi.org/10.1523/JNEUROSCI.4734-03.2004
Maguire JL, Stell BM, Rafizadeh M, Mody I (2005) Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8(6):797–804. https://doi.org/10.1038/nn1469
Maguire J, Mody I (2008) GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron 59(2):207–213. https://doi.org/10.1016/j.neuron.2008.06.019
Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E et al (2009) Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci 29(6):1755–1765. https://doi.org/10.1523/JNEUROSCI.3684-08.2009
Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M (2001) Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409(6816):88–92. https://doi.org/10.1038/35051086
Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A 100(24):14439–14444. https://doi.org/10.1073/pnas.2435457100
Caraiscos VB, Newell JG, You-Ten KE, Elliott EM, Rosahl TW, Wafford KA, MacDonald JF, Orser BA (2004) Selective enhancement of tonic GABAergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J Neurosci 24(39):8454–8458. https://doi.org/10.1523/JNEUROSCI.2063-04.2004
Glykys J, Mann EO, Mody I (2008) Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci 28(6):1421–1426. https://doi.org/10.1523/JNEUROSCI.4751-07.2008
MacKenzie G, Maguire J (2014) The role of ovarian hormone-derived neurosteroids on the regulation of GABAA receptors in affective disorders. Psychopharmacology (Berl) 231(17):3333–3342. https://doi.org/10.1007/s00213-013-3423-z
Maguire J, Mody I (2009) Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinol 34(Suppl 1):S84–S90. https://doi.org/10.1016/j.psyneuen.2009.06.019
Rudolph S, Guo C, Pashkovski SL, Osorno T, Gillis WF, Krauss JM, Nyitrai H, Flaquer I et al (2020) Cerebellum-specific deletion of the GABAA receptor δ subunit leads to sex-specific disruption of behavior. Cell Rep 33(5):108338. https://doi.org/10.1016/j.celrep.2020.108338
Morrow AL (2007) Recent developments in the significance and therapeutic relevance of neuroactive steroids--introduction to the special issue. Pharmacol Ther 116(1):1–6. https://doi.org/10.1016/j.pharmthera.2007.04.003
Luscher B, Shen Q, Sahir N (2011) The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16(4):383–406. https://doi.org/10.1038/mp.2010.120
Fogaça MV, Wu M, Li C, Li XY, Picciotto MR, Duman RS (2021) Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol Psychiatry 26(7):3277–3291. https://doi.org/10.1038/s41380-020-00916-y
Chen S, Chen F, Amin N, Ren Q, Ye S, Hu Z, Tan X, Jiang M et al (2022) Defects of parvalbumin-positive interneurons in the ventral dentate gyrus region are implicated depression-like behavior in mice. Brain Behav Immun 99:27–42. https://doi.org/10.1016/j.bbi.2021.09.013
Ji MH, Zhang L, Mao MJ, Zhang H, Yang JJ, Qiu LL (2020) Overinhibition mediated by parvalbumin interneurons might contribute to depression-like behavior and working memory impairment induced by lipopolysaccharide challenge. Behav Brain Res 383:112509. https://doi.org/10.1016/j.bbr.2020.112509
Yin YY, Wang YH, Liu WG, Yao JQ, Yuan J, Li ZH, Ran YH, Zhang LM et al (2021) The role of the excitation: inhibition functional balance in the mPFC in the onset of antidepressants. Neuropharmacol 191:108573. https://doi.org/10.1016/j.neuropharm.2021.108573
Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF (2004) Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61(7):705–713. https://doi.org/10.1001/archpsyc.61.7.705
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA et al (2012) Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 17(11):1130–1142. https://doi.org/10.1038/mp.2011.113
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC (2007) Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64(2):193–200. https://doi.org/10.1001/archpsyc.64.2.193
Croarkin PE, Levinson AJ, Daskalakis ZJ (2011) Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev 35(3):818–825. https://doi.org/10.1016/j.neubiorev.2010.10.002
Sibille E, Morris HM, Kota RS, Lewis DA (2011) GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol 14(6):721–734. https://doi.org/10.1017/S1461145710001616
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17(8):2921–2927. https://doi.org/10.1523/JNEUROSCI.17-08-02921.1997
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G et al (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Sci 329(5994):959–964. https://doi.org/10.1126/science.1190287
Ghosal S, Duman CH, Liu RJ, Wu M, Terwilliger R, Girgenti MJ, Wohleb E, Fogaca MV et al (2020) Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol Dis 134:104669. https://doi.org/10.1016/j.nbd.2019.104669
Chen S, Gao L, Li X, Ye Y (2021) Allopregnanolone in mood disorders: mechanism and therapeutic development. Pharmacol Res 169:105682. https://doi.org/10.1016/j.phrs.2021.105682
Boero G, Porcu P, Morrow AL (2019) Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiol Stress 27(12):100203. https://doi.org/10.1016/j.ynstr.2019.100203
Balan I, Beattie MC, O'Buckley TK, Aurelian L, Morrow AL (2019) Endogenous neurosteroid (3α,5α)3-hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci Rep 9(1):1220. https://doi.org/10.1038/s41598-018-37409-6
Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A (1998) Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A 95(6):3239–3244. https://doi.org/10.1073/pnas.95.6.3239
Schüle C, Romeo E, Uzunov DP, Eser D, di Michele F, Baghai TC, Pasini A, Schwarz M et al (2006) Influence of mirtazapine on plasma concentrations of neuroactive steroids in major depression and on 3alpha-hydroxysteroid dehydrogenase activity. Mol Psychiatry 11(3):261–272. https://doi.org/10.1038/sj.mp.4001782
Uzunova V, Wrynn AS, Kinnunen A, Ceci M, Kohler C, Uzunov DP (2004) Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol 486(1):31–34. https://doi.org/10.1016/j.ejphar.2003.12.002
Wohlfarth KM, Bianchi MT, Macdonald RL (2002) Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci 22(5):1541–1549. https://doi.org/10.1523/JNEUROSCI.22-05-01541.2002
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN et al (2017) Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390(10093):480–489. https://doi.org/10.1016/S0140-6736(17)31264-3
Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ et al (2018) Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392(10152):1058–1070. https://doi.org/10.1016/S0140-6736(18)31551-4
Davidson JR (2010) Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry 71(Suppl E1):e04. https://doi.org/10.4088/JCP.9058se1c.04gry
Thibaut F (2017) Anxiety disorders: a review of current literature. Dialogues Clin Neurosci 19(2):87–88. https://doi.org/10.31887/DCNS.2017.19.2/fthibaut
Littlejohn EL, Boychuk CR (2021) Protein kinase C-dependent effects of neurosteroids on synaptic GABAA receptor inhibition require the δ-subunit. Front Physiol 25(12):742838. https://doi.org/10.3389/fphys.2021.742838
Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M et al (2014) Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc Natl Acad Sci U S A 111(19):7132–7137. https://doi.org/10.1073/pnas.1403285111
Mody I (2019) GABAAR modulator for postpartum depression. Cell 176(1-2):1. https://doi.org/10.1016/j.cell.2018.12.016
Espallergues J, Mamiya T, Vallée M, Koseki T, Nabeshima T, Temsamani J, Laruelle C, Maurice T (2012) The antidepressant-like effects of the 3β-hydroxysteroid dehydrogenase inhibitor trilostane in mice is related to changes in neuroactive steroid and monoamine levels. Neuropharmacol 62(1):492–502. https://doi.org/10.1016/j.neuropharm.2011.09.005
Espallergues J, Givalois L, Temsamani J, Laruelle C, Maurice T (2009) The 3beta-hydroxysteroid dehydrogenase inhibitor trilostane shows antidepressant properties in mice. Psychoneuroendocrinol 34(5):644–659. https://doi.org/10.1016/j.psyneuen.2008.11.003
Costa AM, Gol M, Lucchi C, Biagini G (2023) Antiepileptogenic effects of trilostane in the kainic acid model of temporal lobe epilepsy. Epilepsia 64(5):1376–1389. https://doi.org/10.1111/epi.17561
Lucchi C, Costa AM, Senn L, Messina S, Rustichelli C, Biagini G (2020) Augmentation of endogenous neurosteroid synthesis alters experimental status epilepticus dynamics. Epilepsia 61(9):e129–e134. https://doi.org/10.1111/epi.16654
Meador KJ, Stowe ZN, Brown C, Robalino CP, Matthews AG, Kalayjian LA, Voinescu PE, Gerard EE et al (2022) Prospective cohort study of depression during pregnancy and the postpartum period in women with epilepsy vs control groups. Neurology 99(15):e1573–e1583. https://doi.org/10.1212/WNL.0000000000200958
Bjørk MH, Veiby G, Reiter SC, Berle JØ, Daltveit AK, Spigset O, Engelsen BA, Gilhus NE (2015) Depression and anxiety in women with epilepsy during pregnancy and after delivery: a prospective population-based cohort study on frequency, risk factors, medication, and prognosis. Epilepsia 56(1):28–39. https://doi.org/10.1111/epi.12884
Turner K, Piazzini A, Franza A, Marconi AM, Canger R, Canevini MP (2009) Epilepsy and postpartum depression. Epilepsia 50(Suppl 1):24–27. https://doi.org/10.1111/j.1528-1167.2008.01965.x
Turner K, Piazzini A, Franza A, Fumarola C, Chifari R, Marconi AM, Canevini MP, Canger R (2006) Postpartum depression in women with epilepsy versus women without epilepsy. Epilepsy Behav 9(2):293–297. https://doi.org/10.1016/j.yebeh.2006.06.003
Maguire J, Ferando I, Simonsen C, Mody I (2009) Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci 29(30):9592–9601. https://doi.org/10.1523/JNEUROSCI.2162-09.2009
Guidotti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E (2001) The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5alpha-dihydroprogesterone in psychiatric disorders. Brain Res Rev 37(1-3):110–115. https://doi.org/10.1016/s0165-0173(01)00129-1
Pinna G, Costa E, Guidotti A (2006) Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 186(3):362–372. https://doi.org/10.1007/s00213-005-0213-2
Griffin LD, Mellon SH (1999) Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A 96(23):13512–13517. https://doi.org/10.1073/pnas.96.23.13512
Ma JH, Wang SY, Yu HY, Li DY, Luo SC, Zheng SS, Wan LF, Duan KM (2019) Prophylactic use of ketamine reduces postpartum depression in Chinese women undergoing cesarean section✰. Psychiatry Res 279:252–258. https://doi.org/10.1016/j.psychres.2019.03.026
Yao J, Song T, Zhang Y, Guo N, Zhao P (2020) Intraoperative ketamine for reduction in postpartum depressive symptoms after cesarean delivery: a double-blind, randomized clinical trial. Brain Behav 10(9):e01715. https://doi.org/10.1002/brb3.1715
Pham TH, Gardier AM (2019) Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacol Ther 199:58–90. https://doi.org/10.1016/j.pharmthera.2019.02.017
Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, Luscher B (2016) Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry 80(6):457–468. https://doi.org/10.1016/j.biopsych.2016.02.009
Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, Taylor SR, Duman CH et al (2020) GABA interneurons are the cellular trigger for ketamine's rapid antidepressant actions. J Clin Invest 130(3):1336–1349. https://doi.org/10.1172/JCI130808
Donegan JJ, Lodge DJ (2017) Hippocampal perineuronal nets are required for the sustained antidepressant effect of ketamine. Int J Neuropsychopharmacol 20(4):354–358. https://doi.org/10.1093/ijnp/pyw095
Pothula S, Kato T, Liu RJ, Wu M, Gerhard D, Shinohara R, Sliby AN, Chowdhury GMI et al (2021) Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol Psychiatry 26(9):5097–5111. https://doi.org/10.1038/s41380-020-0796-3
Zhang B, Yang X, Ye L, Liu R, Ye B, Du W, Shen F, Li Q et al (2021) Ketamine activated glutamatergic neurotransmission by GABAergic disinhibition in the medial prefrontal cortex. Neuropharmacol 15(194):108382. https://doi.org/10.1016/j.neuropharm.2020.108382
Wang DS, Penna A, Orser BA (2017) Ketamine increases the function of γ-aminobutyric acid type A receptors in hippocampal and cortical neurons. Anesthesiol 126(4):666–677. https://doi.org/10.1097/ALN.0000000000001483
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H (2018) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554(7692):317–322. https://doi.org/10.1038/nature25509
Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, Ma S, Sang K et al (2018) Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554(7692):323–327. https://doi.org/10.1038/nature25752
Acknowledgements
The authors would like to thank SaiYing Wang of the Third Xiangya Hospital of Central South University for helpful discussions on topics related to this work. We thank the editor and the reviewers for their useful feedback that improved this paper.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yun Fei Feng, Kai Ming Duan, and Yin Yong Zhou. The first draft of the manuscript was written by Yun Fei Feng and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This is a review. No ethical approval is required.
Consent to Participate
This is a review. There were no human subjects in the study.
Consent for Publication
There are no human subjects, and this review has been approved for publication by all authors.
Competing Interests
The authors declare no competing interests.
We certify that this article is not under consideration for publication elsewhere, and its publication is approved by all authors. If accepted, it will not be published elsewhere in the same form in any other language.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y.F., Zhou, Y.Y. & Duan, K.M. The Role of Extrasynaptic GABA Receptors in Postpartum Depression. Mol Neurobiol 61, 385–396 (2024). https://doi.org/10.1007/s12035-023-03574-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03574-7