Abstract
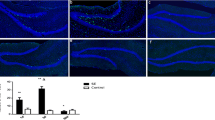
Neural precursors in the subgranular zone (SGZ) can be stimulated by status epilepticus (SE) and ectopically migrate to the hilus. These mislocated cells serve as “potential pacemakers” of spontaneous recurrent seizures, and targeting them could potentially reverse the seizure process. Disrupted-in-Schizophrenia 1 (DISC1) regulates hippocampal neurogenesis after seizures both in vitro and in vivo. Our previous study found that DISC1 was colocalized with neural precursors in the hilus after SE. However, its molecular mechanism and pathways contribute to the ectopic migration of neural precursors to the hilus induced by SE awaits exploration. Here, we showed that both Reelin-ApoER2/EphB2 and Reelin-Integrin β1/Integrin α5 axes may participate in the modulation of neurogenesis after SE. Especially, DISC1, as a protective role, might partly reversed the ectopic progenitor migration via EphB2 pathway. Our findings demonstrated that DISC1 played a protective role in the ectopic migration of neural precursors induced by SE insults and DISC1 could be an attractive new target for the treatment of epilepsy.







Similar content being viewed by others
Data Availability
All data generated or analyzed in this study are available in the published article.
Code Availability
Not applicable.
References
Beghi E (2020) The epidemiology of Epilepsy. Neuroepidemiology 54(2):185–191
Yang C et al (2022) Cadherins and the pathogenesis of epilepsy. Cell Biochem Funct 40(4):336–348
Jessberger S, Parent JM (2015) Epilepsy and adult neurogenesis. Cold Spring Harb Perspect Biol 7(12):a020677
Zhao CS, Overstreet-Wadiche L (2008) Integration of adult generated neurons during epileptogenesis. Epilepsia 49(Suppl 5):3–12
Hester MS, Danzer SC (2013) Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci 33(21):8926–8936
Parent JM, Kron MM (2011) Neurogenesis and epilepsy. Epilepsia 51(Supplement s5):45–45
Scharfman HE (2019) The dentate gyrus and temporal lobe Epilepsy: an “Exciting” era. Epilepsy Curr 19(4):249–255
Althaus AL et al (2015) Intrinsic neurophysiological properties of hilar ectopic and normotopic dentate granule cells in human temporal lobe epilepsy and a rat model. J Neurophysiol 113(4):1184–1194
Duan X et al (2008) Development of neural stem cell in the adult brain. Curr Opin Neurobiol 18(1):108–115
Ye F et al (2017) DISC1 regulates neurogenesis via modulating Kinetochore attachment of Ndel1/Nde1 during mitosis. Neuron 96(5):1041–1054
Bradshaw NJ, Porteous DJ (2012) DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology 62(3):1230–1241
Lee H et al (2015) DISC1-mediated dysregulation of adult hippocampal neurogenesis in rats. Front Syst Neurosci 9:93
Duan X et al (2007) Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130(6):1146–1158
Fournier NM et al (2010) The effect of amygdala kindling on hippocampal neurogenesis coincides with decreased reelin and DISC1 expression in the adult dentate gyrus. Hippocampus 20(5):659–671
Fournier NM et al (2009) Decreased levels of disrupted-in-schizophrenia 1 (DISC1) are associated with expansion of the dentate granule cell layer in normal and kindled rats. Neurosci Lett 455(2):134–139
Jossin Y (2020) Reelin functions, mechanisms of action and signaling pathways during brain development and maturation. Biomolecules 10(6):964
Sekine K et al (2012) Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin alpha5beta1. Neuron 76(2):353–369
Bouché, E et al (2013) Reelin induces EphB activation. Cell Res 23(4):473–490
Hirota Y et al (2018) ApoER2 controls not only neuronal Migration in the Intermediate Zone but also termination of Migration in the developing cerebral cortex. Cereb Cortex 28(1):223–235
Stanfield BB (1979) The development of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol 185(3):423–459
Zhao S et al (2004) Reelin is a positional signal for the lamination of dentate granule cells. Development 131(20):5117–5125
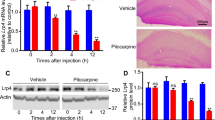
Chen L et al (2022) Disrupted in Schizophrenia 1 regulates ectopic neurogenesis in the mouse Hilus after Pilocarpine-induced Status Epilepticus. Neuroscience 494:69–81
Manseau F, Williams S (2017) Tuning in the hippocampal theta band in vitro: methodologies for recording from the isolated rodent septohippocampal circuit. J Vis Exp (126):55851
Dennis CV et al (2016) Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol Appl Neurobiol 42(7):621–638
Shahsavani M et al (2018) An in vitro model of lissencephaly: expanding the role of DCX during neurogenesis. Mol Psychiatry 23(7):1674–1684
Gong C et al (2007) Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci 27(8):1803–1811
Namba T et al (2011) NMDA receptor regulates migration of newly generated neurons in the adult hippocampus via Disrupted-In-Schizophrenia 1 (DISC1). J Neurochem 118(1):34–44
Wu Q et al (2017) DISC1 regulates the Proliferation and Migration of mouse neural Stem/Progenitor cells through Pax5, Sox2, Dll1 and Neurog2. Front Cell Neurosci 11:261
Althaus AL et al (2019) Altered synaptic drive onto Birthdated Dentate Granule cells in experimental temporal lobe Epilepsy. J Neurosci 39(38):7604–7614
Scharfman H et al (2007) Ectopic granule cells of the rat dentate gyrus. Dev Neurosci 29(1–2):14–27
Dai Y et al (2021) In vivo microelectrode arrays for detecting multi-region epileptic activities in the Hippocampus in the latent period of rat model of temporal lobe Epilepsy. Micromachines (Basel) 12(6):659
Feng BH et al (2021) Expression of disrupted-in-schizophrenia 1 protein and related inflammatory factors inflammatory factors in refractory temporal lobe epilepsy in children. J Biol Regul Homeost Agents 35:221–226
Bosch C et al (2016) Reelin regulates the maturation of dendritic spines, synaptogenesis and glial ensheathment of Newborn Granule cells. Cereb Cortex 26(11):4282–4298
Machado RA et al (2019) Reelin, tau phosphorylation and psychiatric complications in patients with hippocampal sclerosis and structural abnormalities in temporal lobe epilepsy. Epilepsy Behav 96:192–199
Haas CA et al (2002) Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci 22(14):5797–5802
Bradshaw NJ et al (2020) Disrupted in Schizophrenia 1 regulates the processing of reelin in the perinatal cortex. Schizophr Res 215:506–513
Senturk A et al (2011) Ephrin bs are essential components of the Reelin pathway to regulate neuronal migration. Nature 472(7343):356–360
Bouvier D et al (2008) Pre-synaptic and post-synaptic localization of EphA4 and EphB2 in adult mouse forebrain. J Neurochem 106(2):682–695
Grunwald IC et al (2001) Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron 32(6):1027–1040
Davy A, Soriano P (2005) Ephrin signaling in vivo: look both ways. Dev Dyn 232(1):1–10
Chumley MJ et al (2007) EphB receptors regulate Stem/Progenitor cell proliferation, Migration, and polarity during hippocampal neurogenesis. J Neurosci 27(49):13481–13490
Catchpole T, Henkemeyer M (2011) EphB2 tyrosine kinase-dependent forward signaling in migration of neuronal progenitors that populate and form a distinct region of the dentate niche. J Neurosci 31(32):11472–11483
Campbell ID, Humphries MJ (2011) Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3(3):a004994
Tharmalingam S et al (2016) The calcium-sensing receptor and integrins modulate cerebellar granule cell precursor differentiation and migration. Dev Neurobiol 76(4):375–389
Lilja J, Ivaska J (2018) Integrin activity in neuronal connectivity. J Cell Sci 131(12):jcs212803
Brooker SM et al (2016) beta1-integrin restricts astrocytic differentiation of adult hippocampal neural stem cells. Glia 64(7):1235–1251
Izumi Y et al (2017) Integrin alpha5beta1 expression on dopaminergic neurons is involved in dopaminergic neurite outgrowth on striatal neurons. Sci Rep 7:42111
Fasen K et al (2003) Distribution of alpha and beta integrin subunits in the adult rat hippocampus after pilocarpine-induced neuronal cell loss, axonal reorganization and reactive astrogliosis. Acta Neuropathol 106(4):319–322
Jaudon F et al (2021) Integrin adhesion in brain assembly: from molecular structure to neuropsychiatric disorders. Eur J Neurosci 53(12):3831–3850
Wu X et al (2017) Atomic force microscopy investigations of fibronectin and alpha5beta1-integrin signaling in neuroplasticity and seizure susceptibility in experimental epilepsy. Epilepsy Res 138:71–80
Funding
This work was supported by the National Natural Science Foundation of China (82160260, 81601134 to Q.W, 82160261 to Y. H. ), the Yunnan Health Training Project of High-Level Talents (H-2018056 to Q.W., L-2019019 to Y. H.), the Young and Middle-aged Academic and Technical Leaders Reserve Talents Project of Yunnan Province Science and Technology Department (202205AC160019 to Q.W.), Yunnan Applied Basic Research Projects (202201AY070001-083 to Q.W.), and the Scientific Research Fund Project of the Education Department of Yunnan Province (2022Y218 to L.C.).
Author information
Authors and Affiliations
Contributions
Qian Wu: conceived and designed experiments. Lu Chen, Jing Xu, Lin Zhu, and Puying Xu: performed experiments and analyzed the data. Lu Chen, Jing Xu, and Lin Zhu: wrote the paper and final approval of the manuscript. Lu Chen, Puying Xu, and Lvhua Chang: prepared the figures. Qian Wu, Yanbing Han and Lu Chen: responded funding. Qian Wu, Yanbing Han, and Lvhua Chang: critically reviewed and commented on the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal-based experiments were according to the requirements of the Chinese Animal Ethics Committee and approved by Kunming Medical University.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to publication of this manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1) DISC1 might partly reverse the ectopic migration of neural precursors to the hilus via EphB2 pathway after SE;
2) Both Reelin-ApoER2/EphB2 and Reelin-Integrin β1/Integrin α5 axes may participate in the modulation of neurogenesis after SE;
3) DISC1 may play a protective role in the neurogenesis response to SE.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary Fig. 1
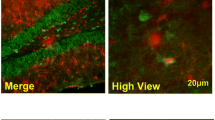
Expression of DISC1 and DCX in the hilus. After SE, DCX + cells increased in the SGZ and migrated ectopically to the hilus. No co-localization of DISC1 and DCX was observed. Green fluorescence labels DISC1, red fluorescence labels DCX, and blue fluorescence labels the nucleus. The white arrows show ectopically migrated DCX + cells, scale bar = 20 μm. (PNG 5.73 MB)

Supplementary Fig. 2
Co-expression of DISC1 and Reelin in the hilus. DISC1 co-localized with Reelin in the SGZ and the hilus. The white arrows show DISC1+/ Reelin + cells, red fluorescence labels DISC1, green fluorescence labels Reelin, and blue fluorescence labels the nucleus, scale bar = 20 μm. (PNG 3.42 MB)

Supplementary Fig. 3
Co-expression of DISC1 and EphB2 in the hilus. DISC1 co-localized with EphB2 in the SGZ and the hilus. The white arrows show DISC1+/ EphB2 + cells, red fluorescence labels DISC1, green fluorescence labels EphB2, and blue fluorescence labels the nucleus, scale bar = 20 μm. (PNG 3.98 MB)

Supplementary Fig. 4
Co-expression of DISC1 and Integrin β1 in the hilus. DISC1 co-localized with Integrin β1 in the GCL, SGZ, and the hilus. The white arrows show DISC1+/ Integrin β1 + cells, red fluorescence labels DISC1, green fluorescence labels Integrin β1, and blue fluorescence labels the nucleus, scale bar = 20 μm. (PNG 6.02 MB)

Supplementary Fig. 5
Co-expression of DISC1 and Integrin α5 in the hilus. DISC1 co-localized with Integrin α5 in the GCL, SGZ, and the hilus. The white arrows show DISC1+/ Integrin α5 + cells, red fluorescence labels DISC1, green fluorescence labels Integrin α5, and blue fluorescence labels the nucleus, scale bar = 20 μm. (PNG 5.73 MB)

Supplementary Fig. 6
Co-expression of DISC1 and Reelin in the hilus after DISC1 up-regulation. DISC1 co-localized with Reelin in the GCL, SGZ, and the hilus. The white arrows show DISC1+/ Reelin + cells, red fluorescence labels DISC1, green fluorescence labels Reelin, and blue fluorescence labels the nucleus, scale bar = 20 μm. (PNG 1.94 MB)

Supplementary Fig. 7
Co-expression of DISC1 and EphB2 in the hilus after DISC1 up-regulation. DISC1 co-localized with EphB2 in the GCL, SGZ, and the hilus. The white arrows show DISC1+/ EphB2 + cells, red fluorescence labels DISC1, green fluorescence labels EphB2, and blue fluorescence labels the nucleus, scale bar = 20 μm. (PNG 2.29 MB)

Supplementary Fig. 8
Co-expression of DISC1 and Integrin β1 in the hilus after DISC1 up-regulation. DISC1 co-localized with Integrin β1 in the GCL, SGZ, and the hilus. The white arrows show DISC1+/ Integrin β1 + cells, red fluorescence labels DISC1, green fluorescence labels Integrin β1, and blue fluorescence labels the nucleus, scale bar = 20 μm. (PNG 2.49 MB)

Supplementary Fig. 9
Co-expression of DISC1 and Integrin α5 in the hilus after DISC1 up-regulation. DISC1 co-localized with Integrin α5 in the GCL, SGZ, and the hilus. The white arrows show DISC1+/ Integrin α5 + cells, red fluorescence labels DISC1, green fluorescence labels Integrin α5, and blue fluorescence labels the nucleus, scale bar = 20 μm. (PNG 2.15 MB)
ESM 10
(DOCX 18.9 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Xu, J., Zhu, L. et al. Disrupted in Schizophrenia 1 Reverse Ectopic Migration of Neural Precursors in Mouse Hilus After Pilocarpine-Induced Status Epilepticus. Mol Neurobiol 60, 6689–6703 (2023). https://doi.org/10.1007/s12035-023-03507-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03507-4