Abstract
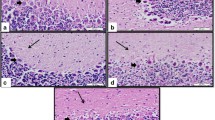
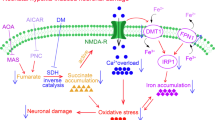
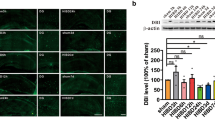
Hypoxic-ischemic encephalopathy is the main cause of infant brain damage, perinatal death, and chronic neonatal disability worldwide. Ferroptosis is a new form of cell death that is closely related to hypoxia-induced brain damage. N-Acetyl serotonin (NAS) exerts neuroprotective effects, but its effects and underlying mechanisms in hypoxia-induced brain damage remain unclear. In the present study, 5-day-old neonatal Sprague–Dawley rats were exposed to hypoxia for 7 days to establish a hypoxia model. Histochemical staining was used to measure the effects of hypoxia on the rat hippocampus. The hippocampal tissue in the hypoxia group showed significant atrophy. Hypoxia significantly increased the levels of prostaglandin-endoperoxide synthase 2 (PTGS2) and the iron metabolism-related protein transferrin receptor 1 (TfR1) and decreased the levels of glutathione peroxidase 4 (GPX4). These changes resulted in mitochondrial damage, causing neuronal ferroptosis in the hippocampus. More importantly, NAS may improve mitochondrial function and alleviate downstream ferroptosis and damage to the hippocampus following hypoxia. In conclusion, we found that NAS could suppress neuronal ferroptosis in the hippocampus following hypoxic brain injury. These discoveries highlight the potential use of NAS as a treatment for neuronal damage through the suppression of ferroptosis, suggesting new treatment strategies for hypoxia-induced brain damage.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Odd D, Heep A, Luyt K, Draycott T (2017) Hypoxic-ischemic brain injury: planned delivery before intrapartum events. J Neonatal Perinatal Med 10(4):347–353. https://doi.org/10.3233/NPM-16152
Cotten CM, Shankaran S (2010) Hypothermia for hypoxic-ischemic encephalopathy. Expert Rev Obstet Gynecol 5(2):227–239. https://doi.org/10.1586/eog.10.7
Shalak L, Perlman JM (2004) Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev 80(2):125–141. https://doi.org/10.1016/j.earlhumdev.2004.06.003
Albertsson AM, Zhang X, Vontell R, Bi D, Bronson RT, Supramaniam V, Baburamani AA, Hua S et al (2018) gammadelta T cells contribute to injury in the developing brain. Am J Pathol 188(3):757–767. https://doi.org/10.1016/j.ajpath.2017.11.012
Novak CM, Ozen M, Burd I (2018) Perinatal brain injury: mechanisms, prevention, and outcomes. Clin Perinatol 45(2):357–375. https://doi.org/10.1016/j.clp.2018.01.015
Volpe JJ (2001) Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev 7(1):56–64. https://doi.org/10.1002/1098-2779(200102)7:1%3c56::AID-MRDD1008%3e3.0.CO;2-A
Cooper JM, Gadian DG, Jentschke S, Goldman A, Munoz M, Pitts G, Banks T, Chong WK et al (2015) Neonatal hypoxia, hippocampal atrophy, and memory impairment: evidence of a causal sequence. Cereb Cortex 25(6):1469–1476. https://doi.org/10.1093/cercor/bht332
Gutziet O, Iluz R, Ben Asher H, Segal L, Ben Zvi D, Ginsberg Y, Khatib N, Zmora O, et al. (2021) Maternal N-acetyl-cysteine prevents neonatal hypoxia-induced brain injury in a rat model. Int J Mol Sci 22 (24). https://doi.org/10.3390/ijms222413629
Roumes H, Dumont U, Sanchez S, Mazuel L, Blanc J, Raffard G, Chateil JF, Pellerin L et al (2021) Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J Cereb Blood Flow Metab 41(2):342–358. https://doi.org/10.1177/0271678X20908355
Gou Z, Su X, Hu X, Zhou Y, Huang L, Fan Y, Li J, Lu L (2020) Melatonin improves hypoxic-ischemic brain damage through the Akt/Nrf2/Gpx4 signaling pathway. Brain Res Bull 163:40–48. https://doi.org/10.1016/j.brainresbull.2020.07.011
Wang ZW, Yang LJ, Ding YX, Chang YZ, Cui H (2016) Insights into the role of iron in immature rat model of hypoxic-ischemic brain injury. Exp Ther Med 12(3):1723–1731. https://doi.org/10.3892/etm.2016.3550
Xu B, Xiao AJ, Chen W, Turlova E, Liu R, Barszczyk A, Sun CLF, Liu L et al (2016) Neuroprotective effects of a PSD-95 inhibitor in neonatal hypoxic-ischemic brain injury. Mol Neurobiol 53(9):5962–5970. https://doi.org/10.1007/s12035-015-9488-4
Huang R, Zhang J, Ren C, Zhang X, Gu L, Dong Y, Zhang J, Zhang J (2019) Effect of erythropoietin on Fas/FasL expression in brain tissues of neonatal rats with hypoxic-ischemic brain damage. NeuroReport 30(4):262–268. https://doi.org/10.1097/WNR.0000000000001194
Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y et al (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262-1279 e1225. https://doi.org/10.1016/j.cell.2019.03.032
Kenny EM, Fidan E, Yang Q, Anthonymuthu TS, New LA, Meyer EA, Wang H, Kochanek PM et al (2019) Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit Care Med 47(3):410–418. https://doi.org/10.1097/CCM.0000000000003555
Leaden PJ, Catala A (2007) Melatonin and N-acetyl serotonin inhibit selectively enzymatic and non-enzymatic lipid peroxidation of rat liver microsomes. Prostaglandins Leukot Essent Fatty Acids 77(1):29–35. https://doi.org/10.1016/j.plefa.2007.06.001
Shen J, Ghai K, Sompol P, Liu X, Cao X, Iuvone PM, Ye K (2012) N-Acetyl serotonin derivatives as potent neuroprotectants for retinas. Proc Natl Acad Sci U S A 109(9):3540–3545. https://doi.org/10.1073/pnas.1119201109
Wolfler A, Abuja PM, Schauenstein K, Liebmann PM (1999) N-Acetylserotonin is a better extra- and intracellular antioxidant than melatonin. FEBS Lett 449(2–3):206–210. https://doi.org/10.1016/s0014-5793(99)00435-4
Zayachkivsky A, Lehmkuhle MJ, Ekstrand JJ, Dudek FE (2015) Ischemic injury suppresses hypoxia-induced electrographic seizures and the background EEG in a rat model of perinatal hypoxic-ischemic encephalopathy. J Neurophysiol 114(5):2753–2763. https://doi.org/10.1152/jn.00796.2014
Yazdani A, Howidi B, Shi MZ, Tugarinov N, Khoja Z, Wintermark P (2021) Sildenafil improves hippocampal brain injuries and restores neuronal development after neonatal hypoxia-ischemia in male rat pups. Sci Rep 11(1):22046. https://doi.org/10.1038/s41598-021-01097-6
Chavez-Valdez R, Emerson P, Goffigan-Holmes J, Kirkwood A, Martin LJ, Northington FJ (2018) Delayed injury of hippocampal interneurons after neonatal hypoxia-ischemia and therapeutic hypothermia in a murine model. Hippocampus 28(8):617–630. https://doi.org/10.1002/hipo.22965
Duran-Carabali LE, Arcego DM, Odorcyk FK, Reichert L, Cordeiro JL, Sanches EF, Freitas LD, Dalmaz C et al (2018) Prenatal and early postnatal environmental enrichment reduce acute cell death and prevent neurodevelopment and memory impairments in rats submitted to neonatal hypoxia ischemia. Mol Neurobiol 55(5):3627–3641. https://doi.org/10.1007/s12035-017-0604-5
Salas J, Reddy N, Orru E, Carson KA, Chavez-Valdez R, Burton VJ, Stafstrom CE, Northington FJ et al (2019) The role of diffusion tensor imaging in detecting hippocampal injury following neonatal hypoxic-ischemic encephalopathy. J Neuroimaging 29(2):252–259. https://doi.org/10.1111/jon.12572
Rosenbaum JL, Almli CR, Yundt KD, Altman DI, Powers WJ (1997) Higher neonatal cerebral blood flow correlates with worse childhood neurologic outcome. Neurology 49(4):1035–1041. https://doi.org/10.1212/wnl.49.4.1035
Vannucci RC (1990) Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res 27(4 Pt 1):317–326. https://doi.org/10.1203/00006450-199004000-00001
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285. https://doi.org/10.1016/j.cell.2017.09.021
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G (2020) Ferroptosis: past, present and future. Cell Death Dis 11(2):88. https://doi.org/10.1038/s41419-020-2298-2
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X (2015) Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59(2):298–308. https://doi.org/10.1016/j.molcel.2015.06.011
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13(1):81–90. https://doi.org/10.1038/nchembio.2238
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379. https://doi.org/10.1038/cdd.2015.158
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T (2017) Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol 403:143–170. https://doi.org/10.1007/82_2016_508
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17. https://doi.org/10.1016/j.redox.2017.01.021
Potter M, Rosenkrantz T, Fitch RH (2018) Behavioral and neuroanatomical outcomes in a rat model of preterm hypoxic-ischemic brain injury: effects of caffeine and hypothermia. Int J Dev Neurosci 70:46–55. https://doi.org/10.1016/j.ijdevneu.2018.02.001
Wang X (2009) The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci Ther 15(4):345–357. https://doi.org/10.1111/j.1755-5949.2009.00105.x
Zhang Y, Cook A, Kim J, Baranov SV, Jiang J, Smith K, Cormier K, Bennett E et al (2013) Melatonin inhibits the caspase-1/cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 55:26–35. https://doi.org/10.1016/j.nbd.2013.03.008
Pandya RS, Zhu H, Li W, Bowser R, Friedlander RM, Wang X (2013) Therapeutic neuroprotective agents for amyotrophic lateral sclerosis. Cell Mol Life Sci 70(24):4729–4745. https://doi.org/10.1007/s00018-013-1415-0
Oxenkrug G, Requintina P, Bachurin S (2001) Antioxidant and antiaging activity of N-acetylserotonin and melatonin in the in vivo models. Ann N Y Acad Sci 939:190–199. https://doi.org/10.1111/j.1749-6632.2001.tb03626.x
Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, Ye K (2010) N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A 107(8):3876–3881. https://doi.org/10.1073/pnas.0912531107
Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D (2011) Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest 121(5):1846–1857. https://doi.org/10.1172/JCI43992
de Moraes JK, Wagner VP, Fonseca FP, Vargas PA, de Farias CB, Roesler R, Martins MD (2018) Uncovering the role of brain-derived neurotrophic factor/tyrosine kinase receptor B signaling in head and neck malignancies. J Oral Pathol Med 47(3):221–227. https://doi.org/10.1111/jop.12611
Funding
This work was supported by the Shandong Provincial Natural Science Foundation of China (Grant No. ZR2015PH036), the China Scholarship Council (Grant No. 201706225023), and the China Postdoctoral Science Foundation (Grant No. 2021M691944). Xiaomei Yang has received research support from the Shandong Provincial Natural Science Foundation of China, the China Scholarship Council and the China Postdoctoral Science Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, investigation, data collection, and analysis were performed by Xiaomei Yang, Yue Yang, Feng Gao, and Kangping Lu. Project administration and supervision was performed by Chunling Wang. The first draft of the manuscript was written by Chunling Wang and Yue Yang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The animal study protocol was approved by the Ethics Committee of Qilu Hospital of Shandong University (protocol code: ECAESDUSM 2012029).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaomei Yang and Yue Yang contributed to the study equally, and both are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Yang, Y., Gao, F. et al. N-Acetyl Serotonin Provides Neuroprotective Effects by Inhibiting Ferroptosis in the Neonatal Rat Hippocampus Following Hypoxic Brain Injury. Mol Neurobiol 60, 6307–6315 (2023). https://doi.org/10.1007/s12035-023-03464-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03464-y