Abstract
Realgar is a traditional Chinese medicine that contains arsenic. It has been reported that the abuse of medicine-containing realgar has potential central nervous system (CNS) toxicity, but the toxicity mechanism has not been elucidated. In this study, we established an in vivo realgar exposure model and selected the end product of realgar metabolism, DMA, to treat SH-SY5Y cells in vitro. Many assays, including behavioral, analytical chemistry, and molecular biology, were used to elucidate the roles of the autophagic flux and the p62-NRF2 feedback loop in realgar-induced neurotoxicity. The results showed that arsenic could accumulate in the brain, causing cognitive impairment and anxiety-like behavior. Realgar impairs the ultrastructure of neurons, promotes apoptosis, perturbs autophagic flux homeostasis, amplifies the p62-NRF2 feedback loop, and leads to p62 accumulation. Further analysis showed that realgar promotes the formation of the Beclin1-Vps34 complex by activating JNK/c-Jun to induce autophagy and recruit p62. Meanwhile, realgar inhibits the activities of CTSB and CTSD and changes the acidity of lysosomes, leading to the inhibition of p62 degradation and p62 accumulation. Moreover, the amplified p62-NRF2 feedback loop is involved in the accumulation of p62. Its accumulation promotes neuronal apoptosis by upregulating the expression levels of Bax and cleaved caspase-9, resulting in neurotoxicity. Taken together, these data suggest that realgar can perturb the crosstalk between the autophagic flux and the p62-NRF2 feedback loop to mediate p62 accumulation, promote apoptosis, and induce neurotoxicity.
Graphical Abstract
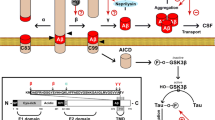
Realgar promotes p62 accumulation to produce neurotoxicity by perturbing the autophagic flux and p62-NRF2 feedback loop crosstalk








Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Liu J, Wei LX, Wang Q, Lu YF, Zhang F, Shi JZ, Li C, Cherian MG (2018) A review of cinnabar (HgS) and/or realgar (As4S4)-containing traditional medicines. J Ethnopharmacol 210:340–350. https://doi.org/10.1016/j.jep.2017.08.037
Wu J, Shao Y, Liu J, Chen G, Ho PC (2011) The medicinal use of realgar (As4S4) and its recent development as an anticancer agent. J Ethnopharmacol 135(3):595–602. https://doi.org/10.1016/j.jep.2011.03.071
Yi Y, Gao SR, Xia J, Li CY, Zhao Y, Zhang YS, Liang AH, Ji S (2020) Study of the accumulation and distribution of arsenic species and association with arsenic toxicity in rats after 30 days of oral realgar administration. J Ethnopharmacol 247:111576. https://doi.org/10.1016/j.jep.2018.10.037
Garza-Lombo C, Pappa A, Panayiotidis MI, Gonsebatt ME, Franco R (2019) Arsenic-induced neurotoxicity: a mechanistic appraisal. J Biol Inorg Chem 24(8):1305–1316. https://doi.org/10.1007/s00775-019-01740-8
Amuno S, Rudko DA, Gallino D, Tuznik M, Shekh K, Kodzhahinchev V, Niyogi S, Chakravarty MM, Devenyi GA (2020) Altered neurotransmission and neuroimaging biomarkers of chronic arsenic poisoning in wildmuskrats (Ondatra zibethicus) and red squirrels (Tamiasciurus hudsonicus) breeding near the City of Yellowknife, Northwest Territories (Canada). Sci Total Environ 707:135556. https://doi.org/10.1016/j.scitotenv.2019.135556
Sharma B, Sharma PM (2013) Arsenic toxicity induced endothelial dysfunction and dementia: Pharmacological interdiction by histone deacetylase and inducible nitric oxide synthase inhibitors. Toxicol Appl Pharmacol 273(1):180–188. https://doi.org/10.1016/j.taap.2013.07.017
Huo T-G, Li W-K, Zhang Y-H, Yuan J, Gao L-Y, Yuan Y, Yang H-L, Jiang H, Sun G-F (2015) Excitotoxicity induced by realgar in the rat hippocampus: the involvement of learning memory injury, dysfunction of glutamate metabolism and NMDA receptors. Mol Neurobiol 51(3):980–994. https://doi.org/10.1007/s12035-014-8753-2
Lu Y, Shi J, Shi J, Liu J (2011) Safety evaluation of realgar-and cinnabar-containing traditional Chinese medicine. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Materia Medica 36(24):3402–5
Garza-Lombó C, Pappa A, Panayiotidis MI, Gonsebatt ME, Franco R (2019) Arsenic-induced neurotoxicity: a mechanistic appraisal. J Biol Inorg Chem : JBIC : Publ Soc Biol Inorg Chem 24(8):1305–1316. https://doi.org/10.1007/s00775-019-01740-8
Meng Y, Feng R, Yang Z, Liu T, Huo T, Jiang H (2022) Oxidative stress induced by realgar in neurons: p38 MAPK and ERK1/2 perturb autophagy and induce the p62-Keap1-Nrf2 feedback loop to activate the Nrf2 signalling pathway. J Ethnopharmacol 282:114582. https://doi.org/10.1016/j.jep.2021.114582
Katsuragi Y, Ichimura Y, Komatsu M (2015) p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J 282(24):4672–4678. https://doi.org/10.1111/febs.13540
Turco E, Witt M, Abert C, Bock-Bierbaum T, Su M-Y, Trapannone R, Sztacho M, Danieli A, Shi X, Zaffagnini G, Gamper A, Schuschnig M, Fracchiolla D, Bernklau D, Romanov J, Hartl M, Hurley JH, Daumke O, Martens S (2019) FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol Cell 74(2):330–346.e11. https://doi.org/10.1016/j.molcel.2019.01.035
Deng Z, Lim J, Wang Q, Purtell K, Wu S, Palomo GM, Tan H, Manfredi G, Zhao Y, Peng J, Hu B, Chen S, Yue Z (2020) ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy 16(5):917–931. https://doi.org/10.1080/15548627.2019.1644076
Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by Nrf2. Antioxid Redox Sig 29(17):1727–1745. https://doi.org/10.1089/ars.2017.7342
Tang Z, Hu B, Zang F, Wang J, Zhang X, Chen H (2019) Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis 10(7):510. https://doi.org/10.1038/s41419-019-1701-3
Zhang YB, Gong JL, Xing TY, Zheng SP, Ding W (2013) Autophagy protein p62/SQSTM1 is involved in HAMLET-induced cell death by modulating apotosis in U87MG cells. Cell Death Dis 4(3):e550. https://doi.org/10.1038/cddis.2013.77
Song C, Charli A, Luo J, Riaz Z, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG (2019) Mechanistic interplay between autophagy and apoptotic signaling in endosulfan-induced dopaminergic neurotoxicity: relevance to the adverse outcome pathway in pesticide neurotoxicity. Toxicol Sci : Off J Soc Toxicol 169(2):333–352. https://doi.org/10.1093/toxsci/kfz049
Jouanne M, Rault S, Voisin-Chiret A-S (2017) Tau protein aggregation in Alzheimer’s disease: an attractive target for the development of novel therapeutic agents. Eur J Med Chem 139:153–167. https://doi.org/10.1016/j.ejmech.2017.07.070
Luo J (2014) Autophagy and ethanol neurotoxicity. Autophagy 10(12):2099–2108. https://doi.org/10.4161/15548627.2014.981916
Rusmini P, Cortese K, Crippa V, Cristofani R, Cicardi ME, Ferrari V, Vezzoli G, Tedesco B, Meroni M, Messi E, Piccolella M, Galbiati M, Garrè M, Morelli E, Vaccari T, Poletti A (2019) Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 15(4):631–651. https://doi.org/10.1080/15548627.2018.1535292
Kumar A, Singh UK, Kini SG, Garg V, Agrawal S, Tomar PK, Pathak P, Chaudhary A, Gupta P, Malik A (2015) JNK pathway signaling: a novel and smarter therapeutic targets for various biological diseases. Future Med Chem 7(15):2065–2086. https://doi.org/10.4155/fmc.15.132
Larhammar M, Huntwork-Rodriguez S, Rudhard Y, Sengupta-Ghosh A, Lewcock JW (2017) The Ste20 family kinases MAP4K4, MINK1, and TNIK converge to regulate stress-induced JNK signaling in neurons. J Neurosci : Off J Soc Neurosci 37(46):11074–11084. https://doi.org/10.1523/JNEUROSCI.0905-17.2017
Tran S, Fairlie WD, Lee EF (2021) BECLIN1: protein structure, function and regulation. Cells 10(6):1522. https://doi.org/10.3390/cells10061522
Lin T, Ruan S, Huang D, Meng X, Li W, Wang B, Zou F (2019) MeHg-induced autophagy via JNK/Vps34 complex pathway promotes autophagosome accumulation and neuronal cell death. Cell Death Dis 10(6):399. https://doi.org/10.1038/s41419-019-1632-z
Pei J, Wang G, Feng L, Zhang J, Jiang T, Sun Q, Ouyang L (2021) Targeting lysosomal degradation pathways: new strategies and techniques for drug discovery. J Med Chem 64(7):3493–3507. https://doi.org/10.1021/acs.jmedchem.0c01689
Ren H, Wang G (2020) Autophagy and lysosome storage disorders. Adv Exp Med Biol 1207:87–102. https://doi.org/10.1007/978-981-15-4272-5_5
Fraldi A, Klein AD, Medina DL, Settembre C (2016) Brain disorders due to lysosomal dysfunction. Annu Rev Neurosci 39:277–295. https://doi.org/10.1146/annurev-neuro-070815-014031
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T (2013) Object recognition test in mice. Nat Protoc 8(12):2531–2537. https://doi.org/10.1038/nprot.2013.155
Kraeuter A-K, Guest PC, Sarnyai Z (2019) The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol (Clifton, N.J.) 1916:99–103. https://doi.org/10.1007/978-1-4939-8994-2_9
Tang J, Bassham DC (2022) Autophagy during drought: function, regulation, and potential application. Plant J : Cell Mol Biol 109(2):390–401. https://doi.org/10.1111/tpj.15481
Tang Q, Zheng G, Feng Z, Chen Y, Lou Y, Wang C, Zhang X, Zhang Y, Xu H, Shang P, Liu H (2017) Trehalose ameliorates oxidative stress-mediated mitochondrial dysfunction and ER stress via selective autophagy stimulation and autophagic flux restoration in osteoarthritis development. Cell Death Dis 8(10):e3081. https://doi.org/10.1038/cddis.2017.453
Liang C, Feng Z, Manthari RK, Wang C, Han Y, Fu W, Wang J, Zhang J (2020) Arsenic induces dysfunctional autophagy via dual regulation of mTOR pathway and Beclin1-Vps34/PI3K complex in MLTC-1 cells. J Hazard Mater 391:122227. https://doi.org/10.1016/j.jhazmat.2020.122227
Zhou Y-Y, Li Y, Jiang W-Q, Zhou L-F (2015) MAPK/JNK signalling: a potential autophagy regulation pathway. Biosci Rep 35(3):e00199. https://doi.org/10.1042/BSR20140141
Schaaf MBE, Keulers TG, Vooijs MA, Rouschop KMA (2016) LC3/GABARAP family proteins: autophagy-(un)related functions. FASEB J : Off Publ Fed Am Soc Exp Biol 30(12):3961–3978. https://doi.org/10.1096/fj.201600698R
Kageyama S, Gudmundsson SR, Sou Y-S, Ichimura Y, Tamura N, Kazuno S, Ueno T, Miura Y, Noshiro D, Abe M, Mizushima T, Miura N, Okuda S, Motohashi H, Lee J-A, Sakimura K, Ohe T, Noda NN, Waguri S, Eskelinen E-L, Komatsu M (2021) p62/SQSTM1-droplet serves as a platform for autophagosome formation and anti-oxidative stress response. Nat Commun 12(1):16. https://doi.org/10.1038/s41467-020-20185-1
Liu M, Pi H, Xi Y, Wang L, Tian L, Chen M, Xie J, Deng P, Zhang T, Zhou C, Liang Y, Zhang L, He M, Lu Y, Chen C, Yu Z, Zhou Z (2021) KIF5A-dependent axonal transport deficiency disrupts autophagic flux in trimethyltin chloride-induced neurotoxicity. Autophagy 17(4):903–924. https://doi.org/10.1080/15548627.2020.1739444
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. BBA-Mol Cell Res 1865(5):721–733. https://doi.org/10.1016/j.bbamcr.2018.02.010
Liu J, Wang C, Li J, Yu Y, Liu Y, Liu H, Peng Q, Guan X (2021) Autophagy blockage promotes the pyroptosis of ox-LDL-treated macrophages by modulating the p62/Nrf2/ARE axis. J Physiol Biochem 77(3):419–429. https://doi.org/10.1007/s13105-021-00811-2
Valavanidis A, Vlachogianni T, Fiotakis C (2009) 8-hydroxy-2’ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Scie Health Part C Environ Carcinog Ecotoxicol Rev 27(2):120–139. https://doi.org/10.1080/10590500902885684
Wu X, Sun R, Wang H, Yang B, Wang F, Xu H, Chen S, Zhao R, Pi J, Xu Y (2019) Enhanced p62-NRF2 feedback loop due to impaired autophagic flux contributes to arsenic-induced malignant transformation of human keratinocytes. Oxid Med Cell Longev 2019:1038932. https://doi.org/10.1155/2019/1038932
Li Y, Wang D, Xu Y, Liu B, Zheng Y, Yang B, Fan S, Zhi X, Zheng Q, Sun G (2015) Use status and metabolism of realgar in Chinese patent medicine. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2015.03.077
Mirzayans R, Murray D (2020) Do TUNEL and other apoptosis assays detect cell death in preclinical studies? Int J Mol Sci 21(23):9090. https://doi.org/10.3390/ijms21239090
Zheng J, Zhang K, Liu Y, Wang Y (2019) Fatal acute arsenic poisoning by external use of realgar: case report and 30 years literature retrospective study in China. Forensic Sci Int 300:e24–e30. https://doi.org/10.1016/j.forsciint.2019.03.012
Wang Y-L, Chen M, Huo T-G, Zhang Y-H, Fang Y, Feng C, Wang S-Y, Jiang H (2017) Effects of glycyrrhetinic acid on GSH synthesis induced by realgar in the mouse hippocampus: involvement of system, System, MRP-1, and Nrf2. Mol Neurobiol 54(4):3102–3116. https://doi.org/10.1007/s12035-016-9859-5
Ys T, Ns W, Sq M, Wp O, Sm Y (2009) Study on the excretion kinetics of arsenic and its metabolites in realgar in rats. Pharmacol Clin Tradit Chin Med 25(3):4 (Chinese)
Chen L, She R, Jiayan MA, Yan Y, Wang H, Quan LI, Luo N, Zhou J, Hongyue MA (2018) Clinical adverse reactions of realgar. Inf Tradit Chin Med 35(6):17–20 (Chinese)
Buchet JP, Lauwerys R, Roels H (1981) Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health 48(1):71–79. https://doi.org/10.1007/BF00405933
Lin S, Shi Q, Nix FB, Styblo M, Beck MA, Herbin-Davis KM, Hall LL, Simeonsson JB, Thomas DJ (2002) A novel S-adenosyl-L-methionine:arsenic(III) methyltransferase from rat liver cytosol. J Biol Chem 277(13):10795–10803
Del Razo LM, Styblo M, Cullen WR, Thomas DJ (2001) Determination of trivalent methylated arsenicals in biological matrices. Toxicol Appl Pharmacol 174(3):282–293. https://doi.org/10.1006/taap.2001.9226
Petrick JS, Jagadish B, Mash EA, Aposhian HV (2001) Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol 14(6):651–656
Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H (2000) Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol 163(2):203–207
Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, Levy D, Alam S, Islam M, Parvez F, Ahsan H, Graziano JH (2007) Folic acid supplementation lowers blood arsenic. Am J Clin Nutr 86(4):1202–1209. https://doi.org/10.1093/ajcn
Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ (2000) Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 74(6):289–299
Galluzzi L, Bravo-San Pedro JM, Blomgren K, Kroemer G (2016) Autophagy in acute brain injury. Nat Rev Neurosci 17(8):467–484. https://doi.org/10.1038/nrn.2016.51
Lumkwana D, du Toit A, Kinnear C, Loos B (2017) Autophagic flux control in neurodegeneration: progress and precision targeting-where do we stand? Prog Neurobiol 153:64–85. https://doi.org/10.1016/j.pneurobio.2017.03.006
Moscat J, Diaz-Meco MT (2009) p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 137(6):1001–1004. https://doi.org/10.1016/j.cell.2009.05.023
Hansen M, Rubinsztein DC, Walker DW (2018) Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol 19(9):579–593. https://doi.org/10.1038/s41580-018-0033-y
Kim KH, Lee M-S (2014) Autophagy–a key player in cellular and body metabolism. Nat Rev Endocrinol 10(6):322–337. https://doi.org/10.1038/nrendo.2014.35
Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Sig 26(12):2694–2701. https://doi.org/10.1016/j.cellsig.2014.08.019
Ugland H, Naderi S, Brech A, Collas P, Blomhoff HK (2011) cAMP induces autophagy via a novel pathway involving ERK, cyclin E and Beclin 1. Autophagy 7(10):1199–1211. https://doi.org/10.4161/auto.7.10.16649
Sarkar S (2013) Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans 41(5):1103–1130. https://doi.org/10.1042/BST20130134
Li A, Zhang JY, Xiao X, Wang SS, Wan JB, Chai YS, Li P, Wang YT (2018) Hepatorenal protective effects of medicinal herbs in An-Gong-Niu-Huang Wan (AGNH) against cinnabar- and realgar-induced oxidative stress and inflammatory damage in mice. Food Chem Toxicol 119:445–456. https://doi.org/10.1016/j.fct.2017.11.054
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741. https://doi.org/10.1016/j.cell.2011.10.026
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42. https://doi.org/10.1016/j.cell.2007.12.018
Lawrence RE, Zoncu R (2019) The lysosome as a cellular centre for signalling, metabolism and quality control. Nat Cell Biol 21(2):133–142. https://doi.org/10.1038/s41556-018-0244-7
Mindell JA (2012) Lysosomal acidification mechanisms. Annu Rev Physiol 74:69–86. https://doi.org/10.1146/annurev-physiol-012110-142317
Mort JS, Buttle DJ (1997) Cathepsin B. Int J Biochem Cell Biol 29(5):715–720
Barrett AJ (1979) Cathepsin D: the lysosomal aspartic proteinase. Ciba Found Symp 75:37–50
Jiang T, Harder B, de la Vega MR, Wong PK, Chapman E, Zhang DD (2015) p62 links autophagy and Nrf2 signaling. Free Radical Biol Med 88:199–204. https://doi.org/10.1016/j.freeradbiomed.2015.06.014
Funding
These authors acknowledge the funding support received from the National Natural Science Foundation of China (grant number 81873082) and the National College Student Innovation and Entrepreneurship Training Program in China (grant number 202010159013).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The material preparation, data collection and analysis were performed by Rui Feng, Jieyu Liu, and Zhao Yang. The first draft of the manuscript was written by Rui Feng, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of China Medical University (ethics committee approval numbers: CMU2019109 and CMU2021120).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, R., Liu, J., Yang, Z. et al. Realgar-Induced Neurotoxicity: Crosstalk Between the Autophagic Flux and the p62-NRF2 Feedback Loop Mediates p62 Accumulation to Promote Apoptosis. Mol Neurobiol 60, 6001–6017 (2023). https://doi.org/10.1007/s12035-023-03452-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03452-2