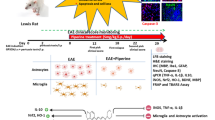
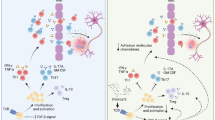
Abstract
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system. Artemisinin (ART) is a natural sesquiterpene lactone with an endoperoxide bond that is well-known for its anti-inflammatory effects in experimental autoimmune encephalomyelitis (EAE), the most commonly used animal model of MS. Tehranolide (TEH) is a novel compound with structural similarity to ART. In this study, we aimed to investigate the ameliorating effect of TEH on EAE development by targeting proteins and genes involved in this process and compare its effects with ART. Female C57BL/6 mice were immunized with MOG35–55. Twelve days post-immunization, mice were treated with 0.28 mg/kg/day TEH and 2.8 mg/kg/day ART for 18 consecutive days, and the clinical score was measured daily. The levels of pro-inflammatory and anti-inflammatory cytokines were assessed in mice serum and splenocytes by ELISA. We also evaluated the mRNA expression level of cytokines, as well as genes involved in T cell differentiation and myelination in the spinal cord tissue by qRT-PCR. Administration of TEH and ART significantly alleviated EAE signs. A significant reduction in IL-6 and IL-17 secretion and IL-17 and IL-1 gene expression in spinal cord were observed in the TEH-treated group. ART had similar or less significant effects. Moreover, TGF-β, IL-4, and IL-10 genes were stimulated by ART and TEH in the spinal cord, while the treatments did not affect IFN-γ expression. Both treatments dramatically increased the expression of FOXP3, GATA3, MBP, and AXL. Additionally, the T-bet gene was reduced after TEH administration. The compounds made no changes in RORγt, nestin, Gas6, Tyro3, and Mertk mRNA expression levels in the spinal cord. The study revealed that both TEH and ART can effectively modulate the genes responsible for inflammation and myelination that play a crucial role in EAE. Interestingly, TEH demonstrated a greater potency compared to ART and hence may have the potential to be evaluated in interventions for the management of MS.







Similar content being viewed by others
Data Availability
The data generated and analyzed during the current study are available from the corresponding author on a reasonable request.
References
Ghasemi N, Razavi S, Nikzad E (2017) Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J 19(1):1–10
Frischer JM et al (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132(Pt 5):1175–1189
Loma I, Heyman R (2011) Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol 9(3):409–416
Nagelkerken L (1998) Role of Th1 and Th2 cells in autoimmune demyelinating disease. Braz J Med Biol Res 31(1):55–60
Raphael I et al (2015) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74(1):5–17
Kaskow BJ, Baecher-Allan C (2018) Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med 8(4):a029025
Das J et al (2009) Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med 206(11):2407–2416
Sutton C et al (2006) A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 203(7):1685–1691
Li Z et al (2015) FOXP3+ regulatory T cells and their functional regulation. Cell Mol Immunol 12(5):558–565
Lee PW, Severin ME, Lovett-Racke AE (2017) TGF-β regulation of encephalitogenic and regulatory T cells in multiple sclerosis. Eur J Immunol 47(3):446–453
Verma ND et al (2021) Multiple sclerosis patients have reduced resting and increased activated CD4+CD25+FOXP3+T regulatory cells. Sci Rep 11(1):10476
Bettelli E et al (2004) Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med 200(1):79–87
Zhang DH et al (1997) Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem 272(34):21597–21603
Jenner RG et al (2009) The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci USA 106(42):17876–17881
Capone A, Volpe E (2020) Transcriptional regulators of T helper 17 cell differentiation in health and autoimmune diseases. Front Immunol 11:348
McDonald WI, Sears TA (1970) The effects of experimental demyelination on conduction in the central nervous system. Brain 93(3):583–598
Martinsen V, Kursula P (2022) Multiple sclerosis and myelin basic protein: insights into protein disorder and disease. Amino Acids 54(1):99–109
Tondo G, Perani D, Comi C (2019) TAM receptor pathways at the crossroads of neuroinflammation and neurodegeneration. Dis Markers 2019:2387614
Goudarzi S et al (2016) Gas6 promotes oligodendrogenesis and myelination in the adult central nervous system and after lysolecithin-induced demyelination. ASN Neuro 8(5):1–14
Shen S et al (2020) The potential of artemisinins as anti-obesity agents via modulating the immune system. Pharmacol Ther 216:107696
Shi C et al (2015) Anti-inflammatory and immunoregulatory functions of artemisinin and its derivatives. Mediat Inflamm 2015:435713
Liu G et al (2022) Artemisinin derivative TPN10466 suppresses immune cell migration and Th1/Th17 differentiation to ameliorate disease severity in experimental autoimmune encephalomyelitis. Cell Immunol 373:104500
Zhang Y et al (2022) Synthesis and biological evaluation of artemisinin derivatives as potential MS agents. Bioorg Med Chem Lett 64:128682
Noori S et al (2010) Tehranolide molecule modulates the immune response, reduce regulatory T cell and inhibits tumor growth in vivo. Mol Immunol 47(7–8):1579–1584
Gajofatto A, Benedetti MD (2015) Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases 3(7):545–555
Mojaverrostami S et al (2018) A review of herbal therapy in multiple sclerosis. Adv Pharm Bull 8(4):575–590
Noori S, Hassan ZM (2012) Tehranolide inhibits proliferation of MCF-7 human breast cancer cells by inducing G0/G1 arrest and apoptosis. Free Radical Biol Med 52(9):1987–1999
Robinson AP et al (2014) The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb Clin Neurol 122:173–189
Constantinescu CS et al (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164(4):1079–1106
Langroudi L et al (2010) A comparison of low-dose cyclophosphamide treatment with artemisinin treatment in reducing the number of regulatory T cells in murine breast cancer model. Int Immunopharmacol 10(9):1055–1061
Bedoya SK et al (2013) Isolation and th17 differentiation of naïve CD4 T lymphocytes. J Vis Exp 79:e50765
Kallaur AP et al (2013) Cytokine profile in relapsing-remitting multiple sclerosis patients and the association between progression and activity of the disease. Mol Med Rep 7(3):1010–1020
Bai Z et al (2019) Cerebrospinal fluid and blood cytokines as biomarkers for multiple sclerosis: a systematic review and meta-analysis of 226 studies with 13,526 multiple sclerosis patients. Front Neurosci 4(13):1026
Moharami S et al (2022) Investigation of serum levels of orexin-A, transforming growth factor β, and leptin in patients with multiple sclerosis. J Clin Lab Anal 36(1):e24170
Jahan-Abad AJ et al (2020) Serum pro-inflammatory and anti-inflammatory cytokines and the pathogenesis of experimental autoimmune encephalomyelitis. Neuropathology 40(1):84–92
Mohajeri M et al (2015) Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 99:156–167
Amedei A, Prisco D, D’Elios MM (2012) Multiple sclerosis: the role of cytokines in pathogenesis and in therapies. Int J Mol Sci 13(10):13438–13460
Mathisen PM et al (1997) Treatment of experimental autoimmune encephalomyelitis with genetically modified memory T cells. J Exp Med 186(1):159–164
Mitchell RE et al (2017) IL-4 enhances IL-10 production in Th1 cells: implications for Th1 and Th2 regulation. Sci Rep 7(1):11315
Chaudhry A et al (2011) Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34(4):566–578
Khakzad MR et al (2017) Artemisinin therapeutic efficacy in the experimental model of multiple sclerosis. Immunopharmacol Immunotoxicol 39(6):348–353
Thomé R et al (2016) Artesunate ameliorates experimental autoimmune encephalomyelitis by inhibiting leukocyte migration to the central nervous system. CNS Neurosci Ther 22(8):707–714
Lv J et al (2021) 9,10-Anhydrodehydroartemisinin Attenuates experimental autoimmune encephalomyelitis by inhibiting Th1 and Th17 cell differentiation. Inflammation 44(5):1793–1802
Li X et al (2013) Artemisinin analogue SM934 ameliorates murine experimental autoimmune encephalomyelitis through enhancing the expansion and functions of regulatory T cell. PLoS ONE 8(8):e74108
Zhao YG et al (2012) Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J Immunol 189(9):4417–4425
Fenimore J, Young HA (2016) Regulation of IFN-γ expression. Adv Exp Med Biol 941:1–19
Huan J et al (2005) Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res 81(1):45–52
Kim HP, Leonard WJ (2007) CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med 204(7):1543–1551
Cheng YH et al (2017) Immunomodulatory effects of Taiwanese Neolitsea species on Th1 and Th2 functionality. J Immunol Res 2017:3529859
Weng WT et al (2021) 4-Ethylguaiacol modulates neuroinflammation and Th1/Th17 differentiation to ameliorate disease severity in experimental autoimmune encephalomyelitis. J Neuroinflammation 18(1):110
Johnson SK et al (2006) The effect of Ginkgo biloba on functional measures in multiple sclerosis: a pilot randomized controlled trial. Explore (NY) 2(1):19–24
Sedighi B et al (2014) Effect of Boswellia papyrifera on cognitive impairment in multiple sclerosis. Iran J Neurol 13(3):149–153
Brady CM et al (2004) An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult Scler 10(4):425–433
Kmietowicz Z (2010) Cannabis based drug is licensed for spasticity in patients with MS. BMJ 340:c3363
Chen S et al (2021) Artemisinin facilitates motor function recovery by enhancing motoneuronal survival and axonal remyelination in rats following brachial plexus root avulsion. ACS Chem Neurosci 12(17):3148–3156
Ran Q et al (2020) Dihydroartemisinin ameliorates murine experimental autoimmune encephalomyelitis through enhancing co-inhibitory signals. Mater Express 10(11):1824–1830
Huang HT et al (2021) Hericium erinaceus mycelium and its small bioactive compounds promote oligodendrocyte maturation with an increase in myelin basic protein. Sci Rep 11(1):6551
Yu H et al (2005) Effect of angelica on proliferation of neural stem cells from rats’ embryos in hypoxia model. Chin J Modern Med 15(21):3226–3228
Binder MD et al (2011) Gas6 increases myelination by oligodendrocytes and its deficiency delays recovery following cuprizone-induced demyelination. PLoS ONE 6(3):e17727
Carrera Silva EA et al (2013) T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity 39(1):160–170
Acknowledgements
We would like to thank Dr. Farshid Noorbakhsh, Professor of immunology, Tehran University of Medical Sciences, for his helpful comments and suggestions. This study was financially supported by Shahid Beheshti University of Medical Sciences; grant number 43002915.
Author information
Authors and Affiliations
Contributions
N.S. performed the experimental work and wrote the manuscript; M.N. critically revised the manuscript; S.N. conceived the study, supervised the research, and analyzed the data. H.R contributed in the conduction of the research and data analysis. A.Z. supervised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animals were treated according to the guidelines for the Care and Use of Laboratory Animals and the experimental procedures were approved by the Animal Use Ethics Committee of Shahid Beheshti University of Medical Science.
Consent to Participate and Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salehi, N., Nourbakhsh, M., Noori, S. et al. Tehranolid and Artemisinin Effects on Ameliorating Experimental Autoimmune Encephalomyelitis by Modulating Inflammation and Remyelination. Mol Neurobiol 60, 5975–5986 (2023). https://doi.org/10.1007/s12035-023-03449-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03449-x