Abstract
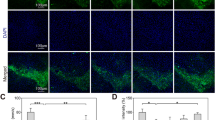
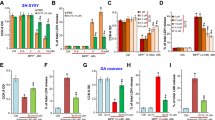
MOTS-c is a 16-amino acid mitochondrial-derived peptide reported to be involved in regulating energy metabolism. However, few studies have reported the role of MOTS-c on neuron degeneration. In this study, it was aimed to explore the action of MOTS-c in rotenone-induced dopaminergic neurotoxicity. In an in vitro study, it was observed that rotenone could influence the expression and localization of MOTS-c significantly in PC12 cells, with more MOTS-c translocating into the nucleus from mitochondria. Further study showed that the translocation of MOTS-c from the mitochondria into the nucleus could directly interact with Nrf2 to regulate HO-1 and NQO1 expression in PC12 cells exposed to rotenone, which had been suggested to be involved in the antioxidant defense system. In vivo and in vitro experiments demonstrated that exogenous MOTS-c pretreatment could protect PC12 cells and rats from mitochondrial dysfunction and oxidative stress induced by rotenone. Moreover, MOTS-c pretreatment significantly decreased the loss of TH, PSD95, and SYP protein expression in the striatum of rats exposed to rotenone. In addition, MOTS-c pretreatment could clearly alleviate the downregulated expression of Nrf2, HO-1, and NQO1, as well as the upregulated Keap1 protein expression in the striatum of rotenone-treated rats. Taken together, these findings suggested that MOTS-c could directly interact with Nrf2 to activate the Nrf2/HO-1/NQO1 signal pathway to defend the antioxidant system to prevent dopaminergic neurons from rotenone-induced oxidative stress and neurotoxicity in vitro and in vivo.







Similar content being viewed by others
Data Availability
All data that support the findings of this study are available from the corresponding author upon request.
References
Adam D (2000) Pesticide use linked to Parkinson’s disease. Nature 408(6809):125. https://doi.org/10.1038/35041740
Bueler H (2010) Mitochondrial dynamics, cell death and the pathogenesis of Parkinson’s disease. Apoptosis 15(11):1336–1353. https://doi.org/10.1007/s10495-010-0465-0
Yasuda T, Hayakawa H, Nihira T, Ren YR, Nakata Y, Nagai M, Hattori N, Miyake K, Takada M, Shimada T, Mizuno Y, Mochizuki H (2011) Parkin-mediated protection of dopaminergic neurons in a chronic MPTP-minipump mouse model of Parkinson disease. J Neuropathol Exp Neurol 70(8):686–697. https://doi.org/10.1097/NEN.0b013e3182269ecd
Merry TL, Chan A, Woodhead JST, Reynolds JC, Kumagai H, Kim SJ, Lee C (2020) Mitochondrial-derived peptides in energy metabolism. Am J Physiol Endocrinol Metab 319(4):E659–E666. https://doi.org/10.1152/ajpendo.00249.2020
Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P (2015) The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21(3):443–454. https://doi.org/10.1016/j.cmet.2015.02.009
Kim KH, Son JM, Benayoun BA, Lee C (2018) The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab 28(3):516–524. https://doi.org/10.1016/j.cmet.2018.06.008
Kim SJ, Mehta HH, Wan J, Kuehnemann C, Chen J, Hu JF, Hoffman AR, Cohen P (2018) Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging (Albany NY) 10(6):1239–1256. https://doi.org/10.18632/aging.101463
D'Souza RF, Woodhead JST, Hedges CP, Zeng N, Wan J, Kumagai H, Lee C, Cohen P, Cameron-Smith D, Mitchell CJ, Merry TL (2020) Increased expression of the mitochondrial derived peptide, MOTS-c, in skeletal muscle of healthy aging men is associated with myofiber composition. Aging (Albany NY) 12(6):5244–5258. https://doi.org/10.18632/aging.102944
Sequeira IR, Woodhead JST, Chan A, D'Souza RF, Wan J, Hollingsworth KG, Plank LD, Cohen P, Poppitt SD, Merry TL (2021) Plasma mitochondrial derived peptides MOTS-c and SHLP2 positively associate with android and liver fat in people without diabetes. Biochim Biophys Acta Gen Subj 1865(11):129991. https://doi.org/10.1016/j.bbagen.2021.129991
Kim GH, Kim JE, Rhie SJ, Yoon S (2015) The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 24(4):325–340. https://doi.org/10.5607/en.2015.24.4.325
Liu C, Gidlund EK, Witasp A, Qureshi AR, Soderberg M, Thorell A, Nader GA, Barany P, Stenvinkel P, von Walden F (2019) Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease. Am J Physiol Renal Physiol 317(5):F1122–F1131. https://doi.org/10.1152/ajprenal.00202.2019
Ramanjaneya M, Jerobin J, Bettahi I, Bensila M, Aye M, Siveen KS, Sathyapalan T, Skarulis M, Abou-Samra AB, Atkin SL (2019) Lipids and insulin regulate mitochondrial-derived peptide (MOTS-c) in PCOS and healthy subjects. Clin Endocrinol (Oxf) 91(2):278–287. https://doi.org/10.1111/cen.14007
Yen K, Lee C, Mehta H, Cohen P (2013) The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol 50(1):R11–R19. https://doi.org/10.1530/JME-12-0203
Lu H, Wei M, Zhai Y, Li Q, Ye Z, Wang L, Luo W, Chen J, Lu Z (2019) MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med (Berl) 97(4):473–485. https://doi.org/10.1007/s00109-018-01738-w
Sreekumar PG, Ishikawa K, Spee C, Mehta HH, Wan J, Yen K, Cohen P, Kannan R, Hinton DR (2016) The mitochondrial-derived peptide humanin protects RPE cells from oxidative stress, senescence, and mitochondrial dysfunction. Invest Ophthalmol Vis Sci 57(3):1238–1253. https://doi.org/10.1167/iovs.15-17053
Woodhead JST, D'Souza RF, Hedges CP, Wan J, Berridge MV, Cameron-Smith D, Cohen P, Hickey AJR, Mitchell CJ (1985) Merry TL (2020) High-intensity interval exercise increases humanin, a mitochondrial encoded peptide, in the plasma and muscle of men. J Appl Physiol 128(5):1346–1354. https://doi.org/10.1152/japplphysiol.00032.2020
Liu J, Chang L, Roselli F, Almeida OF, Gao X, Wang X, Yew DT, Wu Y (2010) Amyloid-beta induces caspase-dependent loss of PSD-95 and synaptophysin through NMDA receptors. J Alzheimers Dis 22(2):541–556. https://doi.org/10.3233/JAD-2010-100948
Wang H, Lv J, Jiang N, Huang H, Wang Q, Liu X (2021) Ginsenoside Re protects against chronic restraint stress-induced cognitive deficits through regulation of NLRP3 and Nrf2 pathways in mice. Phytother Res. https://doi.org/10.1002/ptr.6947
Narasimhan KKS, Jayakumar D, Velusamy P, Srinivasan A, Mohan T, Ravi DB, Uthamaraman S, Sathyamoorthy YK, Rajasekaran NS, Periandavan K (2019) Morinda citrifolia and its active principle scopoletin mitigate protein aggregation and neuronal apoptosis through augmenting the DJ-1/Nrf2/ARE signaling pathway. Oxid Med Cell Longev 2019:2761041. https://doi.org/10.1155/2019/2761041
Zhang H, Davies KJA, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88(Pt B):314–336. https://doi.org/10.1016/j.freeradbiomed.2015.05.036
Kim SJ, Miller B, Kumagai H, Silverstein AR, Flores M, Yen K (2021) Mitochondrial-derived peptides in aging and age-related diseases. Geroscience 43(3):1113–1121. https://doi.org/10.1007/s11357-020-00262-5
Lim CS, Han JS (2018) The antioxidant xanthorrhizol prevents amyloid-beta-induced oxidative modification and inactivation of neprilysin. Biosci Rep 38(1). https://doi.org/10.1042/BSR20171611
Yan Y, Ren Y, Li X, Zhang X, Guo H, Han Y, Hu J (2018) A polysaccharide from green tea (Camellia sinensis L.) protects human retinal endothelial cells against hydrogen peroxide-induced oxidative injury and apoptosis. Int J Biol Macromol 115:600–607. https://doi.org/10.1016/j.ijbiomac.2018.04.011
Mitsuishi Y, Motohashi H, Yamamoto M (2012) The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2:200. https://doi.org/10.3389/fonc.2012.00200
Wei YZ, Zhu GF, Zheng CQ, Li JJ, Sheng S, Li DD, Wang GQ, Zhang F (2020) Ellagic acid protects dopamine neurons from rotenone-induced neurotoxicity via activation of Nrf2 signalling. J Cell Mol Med 24(16):9446–9456. https://doi.org/10.1111/jcmm.15616
Zagoura D, Canovas-Jorda D, Pistollato F, Bremer-Hoffmann S, Bal-Price A (2017) Evaluation of the rotenone-induced activation of the Nrf2 pathway in a neuronal model derived from human induced pluripotent stem cells. Neurochem Int 106:62–73. https://doi.org/10.1016/j.neuint.2016.09.004
Deng C, Cao J, Han J, Li J, Li Z, Shi N, He J (2018) Liraglutide activates the Nrf2/HO-1 antioxidant pathway and protects brain nerve cells against cerebral ischemia in diabetic rats. Comput Intell Neurosci 2018:3094504. https://doi.org/10.1155/2018/3094504
Shen C, Wang J, Feng M, Peng J, Du X, Chu H, Chen X (2021) The mitochondrial-derived peptide MOTS-c attenuates oxidative stress injury and the inflammatory response of H9c2 cells through the Nrf2/ARE and NF-kappaB pathways. Cardiovasc Eng Technol. https://doi.org/10.1007/s13239-021-00589-w
Yang Q, Lin J, Zhang H, Liu Y, Kan M, Xiu Z, Chen X, Lan X, Li X, Shi X, Li N, Qu X (2019) Ginsenoside compound K regulates amyloid beta via the Nrf2/Keap1 signaling pathway in mice with scopolamine hydrobromide-induced memory impairments. J Mol Neurosci 67(1):62–71. https://doi.org/10.1007/s12031-018-1210-3
Jiang J, Chang X, Nie Y, Shen Y, Liang X, Peng Y, Chang M (2021) Peripheral administration of a cell-penetrating MOTS-c analogue enhances memory and attenuates Abeta1-42- or LPS-induced memory impairment through inhibiting neuroinflammation. ACS Chem Neurosci 12(9):1506–1518. https://doi.org/10.1021/acschemneuro.0c00782
Funding
This work was supported by grants from the NSFC (Natural Science Foundation of China) (81973090) to Yan Sai.
Author information
Authors and Affiliations
Contributions
JX contributed to investigation, writing—original draft, and visualization; QF and YH were involved in investigation; FY, XZ, JC, GD, XG, and YZ made software; ML contributed to writing—review and editing; YS performed project administration, conceptualization, supervision, validation, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
Animal care and all experimental procedures were conducted in compliance with the Animal Ethics Committee of the Third Military Medical University. All efforts were made to minimize the number of animals used and their suffering. All experimental procedures were approved by the Ethics Committee of Third Military Medical University. The animal use was conducted in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and by the Animal Ethics Committee of Third Military Medical University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, J., Zhang, Q., Shan, Y. et al. The Mitochondrial-Derived Peptide (MOTS-c) Interacted with Nrf2 to Defend the Antioxidant System to Protect Dopaminergic Neurons Against Rotenone Exposure. Mol Neurobiol 60, 5915–5930 (2023). https://doi.org/10.1007/s12035-023-03443-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03443-3