Abstract
All biological tissues and bodily fluids include the autacoid adenosine. The P1 class of purinergic receptors includes adenosine receptors. Four distinct G-protein-coupled receptors on the cellular membrane mediate the effects of adenosine, whose cytoplasmic content is regulated by producing/degrading enzymes and nucleoside transporters. A2A receptor has received a great deal of attention in recent years because it has a wide range of potential therapeutic uses. A2B and, more significantly, A2A receptors regulate numerous physiological mechanisms in the central nervous system (CNS). The inferior targetability of A2B receptors towards adenosine points that they might portray a promising medicinal target since they are triggered only under pharmacological circumstances (when adenosine levels rise up to micromolar concentrations). The accessibility of specific ligands for A2B receptors would permit the exploration of such a theory. A2A receptors mediate both potentially neurotoxic and neuroprotective actions. Hence, it is debatable to what extent they play a role in neurodegenerative illnesses. However, A2A receptor blockers have demonstrated clear antiparkinsonian consequences, and a significant attraction exists in the role of A2A receptors in other neurodegenerative disorders. Amyloid peptide extracellular accumulation and tau hyperphosphorylation are the pathogenic components of AD that lead to neuronal cell death, cognitive impairment, and memory loss. Interestingly, in vitro and in vivo research has shown that A2A adenosine receptor antagonists may block each of these clinical symptoms, offering a crucial new approach to combat a condition for which, regrettably, only symptomatic medications are currently available. At least two requirements must be met to determine whether such receptors are a target for diseases of the CNS: a complete understanding of the mechanisms governing A2A-dependent processes and the availability of ligands that can distinguish between the various receptor populations. This review concisely summarises the biological effects mediated by A2A adenosine receptors in neurodegenerative disorders and discusses the chemical characteristics of A2A adenosine receptor antagonists undergoing clinical trials.
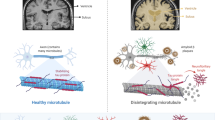
Graphical Abstract
Selective A2A receptor blocker against neurodegenerative disorders







Similar content being viewed by others
Data Availability
Any additional data could be available from the corresponding author upon request.
References
Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J (1998) Immunohistochemical localization of adenosine A 2A receptors in the rat central nervous system. J Comp Neurol 401:163–186
Kondo T, Mizuno Y (2015) Japanese Istradefylline Study Group A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol 38:41–46
Saki M, Yamada K, Koshimura E, Sasaki K, Kanda T (2013) In vitro pharmacological profile of the A2A receptor antagonist istradefylline. Naunyn Schmiedebergs Arch Pharmacol 386:963–972
Mizuno Y, Kondo T (2013) Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord 28:1138–1141
Feigin VL, Vos T, Abajobir AA et al (2017) Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16(11):877–897. https://doi.org/10.1016/S1474-4422(17)30299-5
Standaert DG, Roberson ED (2011) Treatment of central nervous system degenerative disorders. In: Brunton LL (ed) Goodman & Gilman’s: the pharmacological basis of therapeutics, 12th edn. McGraw-Hill, New York, pp 609–628
Fredholm BB (2014) Adenosine—a physiological or pathophysiological agent? J Mol Med 92(3):201–206
Zhang Y, Li P, Feng J, Wu M (2016) Review on dysfunction of NMDA receptors in Alzheimer’s disease. Neurol Sci 37:1039–1047
Liu Z et al (2015) Probe to bifunctional memantine derivatives for treatment of Alzheimer’s disease. J Pharm Biomed Sci 05(04):276–290
Schmitt FA, Wichems CHA (2006) Systematic review of assessment and treatment of moderate to severe Alzheimer’s disease. Prim Care Companion J Clin Psychiatry 8:158–159
Li JH, Vicknasingam B, Cheung YW, Zhou W et al (2011) To use or not to use: an update on licit and illicit ketamine use. Subst Abuse Rehab 2:11–20
Knopman DS, Amieva H, Petersen RC et al (2021) Alzheimer disease. Nat Rev Dis Primers 7(1):33
Bhushan I, Kour M, Kour G, Gupta S (2018) Alzheimer’s disease: causes & treatment – a review. Ann Biotechnol 1(1):1002
Scheltens P, Blennow K, Breter MMB, Strooper BD et al (2016) Alzheimer’s disease. Lancet 388:505–517
Weller J, Budson A (2018) Review on current understanding of Alzheimer’s disease diagnosis and treatment. F1000 Res 7:1–9
Chou E (2014) Alzheimer’s disease: current and future treatments. a review. Int J Med Students 2:56–63
Thies and Bleiler (2013) Alzheimer’s disease facts and figures. Alzheimer Dement 9:208-245
Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T et al (2006) TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature 440:1208–1212. https://doi.org/10.1038/nature04667
Zhang X, Wu Y, Cai F, Song W (2019) Regulation of global gene expression in brain by TMP21. Mol Brain 12:39. https://doi.org/10.1186/s13041-0190460-5
Rozpedek-Kaminska W, Siwecka N, Wawrzynkiewicz A, Wojtczak R, Pytel D, Diehl JA, Majsterek I (2020) The PERK-dependent molecular mechanisms as a novel therapeutic target for neurodegenerative diseases. Int J Mol Sci 21:2108
Koszła O, Stepnicki P, Zieba A, Grudzinska A, Matosiuk D, Kaczor AA (2021) Current approaches and tools used in drug development against Parkinson’s disease. Biomolecules 11:897
Lopes CR, Cunha RA, Agostinho P (2021) Astrocytes and adenosine A2A receptors: active players in Alzheimer’s disease. Front Neurosci 15:666710
Franco R, Navarro G (2018) Adenosine A2A receptor antagonists in neurodegenerative diseases: huge potential and huge challenges. Front Psychiatry 9:68
Jacobson KA (2009) Introduction to adenosine receptors as therapeutic targets. Handb Exp Pharmacol 193:1–24
Bennett KA, Tehan B, Lebon G et al (2013) Pharmacology and structure of isolated conformations of the adenosine A2A receptor define ligand efficacy. Mol Pharmacol 83(5):949–958
Costanzi S, Ivanov AA, Tikhonova IG, Jacobson KA (2007) Structure and function of G protein-coupled receptors studied using sequence analysis, molecular modeling, and receptor engineering: Adenosine receptors. Front Drug Design Disc 3:63–79
Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM (1992) Molecular cloning of rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Mol Brain Res 14:186–190
Fredholm B, Svenningsson P (2003) Adenosine-dopamine interactions. Neurology 61:5–9
Rosin D, Hettinger B, Lee A, Linden J (2003) Anatomy of adenosine A2A receptors in brain. Neurology 61:12–18
Popoli P, Pezzola A, Reggio R, Caporali MG, Scotti de Carolis A (1994) Modulation of striatal adenosine A1 and A2 receptors induces rotation behavior in response to dopaminergic stimulation in rats. Eur J Pharmacol 257:5–6
Mori A, Shindou T (2003) Modulation of GABAergic transmission in the striatopallidal system by adenosine A2A receptors. Neurology 61:44–48
Shindou T, Nonaka H, Richardson PJ, Mori A, Kase H, Ichimura M (2002) Presynaptic adenosine A2A receptors enhance GABAergic synaptic transmission via cyclic AMP dependent mechanism in the rat globus pallidus. Brit J Pharmacol 136:296–302
Preston Z, Lee K, Widdowson L, Freeman TC, Dixon AK, Richardson PJ (2000) Adenosine receptor expression and function in rat striatal cholinergic interneurons. Brit J Pharmacol 130:886–890
Cieślak M, Komoszyński M, Wojtczak A (2008) Adenosine A2A receptors in Parkinson’s disease treatment. Purinergic Signaling 4:305–312
Mandrekar-Colucci S, Karlo JC, Landreth GE (2012) Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J Neurosci 32:10117–10128
Park K, Kim E, Han H, Shim Y, Kwon J, Ku B, Park KH, Yi HA, Kim KK, Yang DW (2017) Efficacy and tolerability of rivastigmine patch therapy in patients with mild-to-moderate Alzheimer’s dementia associated with minimal and moderate ischemic white matter hyperintensities: a multicenter prospective open-label clinical trial. PLoS ONE 12:e0182123
Stoiljkovic M, Horvath TL, Hajes M (2021) Therapy for Alzheimer’s disease: missing targets and functional markers? Ageing Res Rev 68:101318
Johansson M, Stomrud E, Lindberg O, Westman E, Johansson PM, van Westen D, Mattsson N, Hansson O (2020) Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol Aging 85:74–82
Cummings J (2021) New approaches to symptomatic treatments for Alzheimer’s disease. Mol Neurodegener 16:2
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179:312–339
Mullard AFDA (2021) Approval for Biogen’s aducanumab sparks Alzheimer disease firestorm. Nat Rev Drug Discov 20:496
Jacobson KA, Gao ZG, Matricon P, Eddy MT, Carlsson J (2022) Adenosine A2A receptor antagonists: from caffeine to selective non-xanthines. Br J Pharmacol 179(14):1–16
Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA (2002) Adenosine A2A receptor facilitation of hippocampal synaptic transmission is dependent on tonic A1 receptor inhibition. Neuroscience 112:319–329
Gonçalves FQ, Lopes JP, Silva HB, Lemos C, Silva AC, Gonçalves N, Tomé ÂR, Ferreira SG et al (2019) Synaptic and memory dysfunction in a β-amyloid model of early Alzheimer’s disease depends on increased formation of ATP-derived extracellular adenosine. Neurobiol Dis 132:104570
Rebola N, Canas PM, Oliveira CR, Cunha RA (2005) Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience 132:893–903
Rebola N, Lujan R, Cunha RA, Mulle C (2008) Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57:121–134
Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Gro MC, Schwarzschild MA, Chen JF et al (2005) Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J Neurochem 95:1188–1200
Costenla AR, Diógenes MJ, Canas PM, Rodrigues RJ, Nogueira C, Maroco J, Agostinho PM, Ribeiro JA et al (2011) Enhanced role of adenosine A2A receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci 34:12–21
Temido-Ferreira M, Coelho JE, Pousinha PA, Lopes LV (2019) Novel Players in the aging synapse: impact on cognition. J Caffeine Adenosine Res 9:104–127
Pinna A, Serra M, Morelli M, Simola N (2018) Role of adenosine A2A receptors in motor control: relevance to Parkinson’s disease and dyskinesia. J Neural Transm 125:1273–1286. https://doi.org/10.1007/s00702-018-1848-6
Navarro G, Borroto-Escuela DO, Fuxe K, Franco R (2015) Purinergic signaling in Parkinson’s disease. Relevance for treatment. Neuropharmacology 104(2016):161–168. https://doi.org/10.1016/j.neuropharm.2015.07.024
Mori A (2014) Mode of action of adenosine A2A receptor antagonists as symptomatic treatment for Parkinson’s disease. Int Rev Neurobiol 119:87–116. https://doi.org/10.1016/B978-0-12-801022-8.00004-0
Jenner P (2014) An overview of adenosine A2A receptor antagonists in Parkinson’s disease. Int Rev Neurobiol 119:71–86. https://doi.org/10.1016/B978-0-12-801022-8.00003-9
Wardas J, Konieczny J, Lorenc-Koci E (2001) SCH 58261, an A(2A) adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse 41:160–171. https://doi.org/10.1002/syn.1070
Popoli P, Reggio R, Pezzola A (2000) Effects of SCH 58261, an adenosine A(2A) receptor antagonist, on quinpirole-induced turning in 6-hydroxydopamine-lesioned rats. Lack of tolerance after chronic caffeine intake. Neuropsychopharmacology. 22:522–529. S0893–133X(99)00144-X [pii].
Grondin R, Bedard PJ, Tahar AH, Gregoire L, Mori A, Kase H (1999) Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology 52:1673–1677
Hodgson RA, Bedard PJ, Varty GB, Kazdoba TM, Di Paolo T, Grzelak ME, Pond AJ, Hadjtahar A et al (2010) A selective A(2A) receptor antagonist, is active in primate models of movement disorders. Exp Neurol 225:384–390. https://doi.org/10.1016/j.expneurol.2010.07.011[doi]
Franco R, Ferre S, Agnati L, Torvinen M, Gines S, Hillion J, Casado V, Lledo P et al. (2000) Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacology. 23: S50–9. S0893133X00001445
Navarro G, Cordomi A, Casado-Anguera V, Moreno E, Cai NS, Cortes A, Canela EI, Dessauer CW et al (2018) Evidence for functional pre-coupled complexes of receptor heteromers and adenylyl cyclase. Nat Commun 9:1242–1243. https://doi.org/10.1038/s41467-018-03522-3
Pourcher E, Fernandez HH, Stacy M, Mori A, Ballerini R, Chaikin P (2012) Istradefylline for Parkinson’s disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study, Parkinsonism Relat. Disord 18:178–184. https://doi.org/10.1016/j.parkreldis.2011.09.023
Hauser RA, Stocchi F, Rascol O, Huyck SB, Capece R, Ho TW, Sklar P, Lines C et al (2015) Preladenant as an adjunctive therapy with levodopa in Parkinson disease: two randomized clinical trials and lessons learned. JAMA Neurol 72:1491–1500. https://doi.org/10.1001/jamaneurol.2015.2268
Hauser RA, Shulman LM, Trugman JM, Roberts JW, Mori A, Ballerini R, Sussman NM (2008) I. 6002- U.-013 S. Group, Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov Disord 23:2177–2185. https://doi.org/10.1002/mds.22095
Stacy M, Silver D, Mendis T, Sutton J, Mori A, Chaikin P, Sussman NM (2008) A 12-week, placebocontrolled study (6002-US-006) of istradefylline in Parkinson disease. Neurology 70:2233–2240. https://doi.org/10.1212/01.wnl.0000313834.22171.17
LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM (2008) 6002-US-005 Study Group, Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol 63:295–302. https://doi.org/10.1002/ana.21315
Hauser RA, Hubble JP, Truong DD (2003) I.U.-001 S. Group, Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology 61:297–303. https://doi.org/10.1212/01.wnl.0000081227.84197.0b
Zhao J, Kumar M, Sharma J, Yuan Z (2021) Arbutin effectively ameliorates the symptoms of Parkinson’s disease: the role of adenosine receptors and cyclic adenosine monophosphate. Neural Regen Res 16(10):2030–2040
Wang M, Hou S, Wei Y, Li D et al (2021) Discovery of novel dual adenosine A1/A2A receptor antagonists using deep learning, pharmacophore modeling and molecular docking. PLoS Comput Biol 17(3):e1008821
Otte C, Gold SM, Penninx BW et al (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065
Kok RM, Reynolds CF III (2017) Management of depression in older adults: a review. JAMA 317(20):2114–2122
Yamada K, Kobayashi M, Kanda T (2014) Involvement of adenosine A2A receptors in depression and anxiety. Int Rev Neurobiol 119:373–393
Hunter AM, Balleine BW, Minor TR (2003) Helplessness and escape performance: glutamate-adenosine interactions in the frontal cortex. BehavNeurosci 117(1):123–135
Lindquist BE, Shuttleworth CW (2017) Evidence that adenosine contributes to Leao’s spreading depression in vivo. J Cereb Blood Flow Metab 37(5):1656–1669
Gubert C, Jacintho Moritz CE, Vasconcelos-Moreno MP et al (2016) Peripheral adenosine levels in euthymic patients with bipolar disorder. Psychiatry Res 246:421–426
Hart E, Conoscenti M, Minor T (2014) Animal models of depression: a focus on adenosine signaling at A2A receptors. Ann Depress Anxiety 1:1285–1292
Coelho JE, Alves P, Canas PM et al (2014) Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion, and anxiety. Front Psychiatry 5:67
López-Cruz L, Carbó-Gas M, Pardo M et al (2017) Adenosine A2A receptor deletion affects social behaviors and anxiety in mice: involvement of anterior cingulate cortex and amygdala. Behav Brain Res 321:8–17
Wei CJ, Augusto E, Gomes CA et al (2014) Regulation of fear responses by striatal and extrastriatal adenosine A2A receptors in forebrain. Biol Psychiatry 75(11):855–863
Caetano L, Pinheiro H, Patrício P et al (2017) Adenosine A2A receptor regulation of microglia morphological remodeling-gender bias in physiology and in a model of chronic anxiety. Mol Psychiatry 22(7):1035–1043
El Yacoubi M, Costentin J, Vaugeois J-M (2003) Adenosine A2A receptors and depression. Neurology 61(11, Supplement 6):S82-7
Deckert J (1998) The adenosine A(2A) receptor knockout mouse: a model for anxiety? Int J Neuropsychopharmacol 1(2):187–190
Alsene K, Deckert J, Sand P, de Wit H (2003) Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology 28(9):1694–1702
Freitag CM, Agelopoulos K, Huy E, Rothermundt M, Krakowitzky P, Meyer J, Deckert J, von Gontard A et al (2010) Adenosine A(2A) receptor gene (ADORA2A) variants may increase autistic symptoms and anxiety in autism spectrum disorder. Eur Child Adolesc Psychiatry 1:67–74
Deckert J, Nöthen MM, Franke P, Delmo C, Fritze J, Knapp M, Maier W, Beckmann H et al (1998) Systematic mutation screening and association study of the A1 and A2a adenosine receptor genes in panic disorder suggest a contribution of the A2a gene to the development of disease. Mol Psychiatry 1:81–85
Hamilton SP, Slager SL, de Leon AB, Heiman GA, Klein DF, Hodge SE, Weissman MM, Fyer AJ et al (2004) Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology 29(3):558–565
Lam P, Hong CJ, Tsai SJ (2005) Association study of A2a adenosine receptor genetic polymorphism in panic disorder. Neurosci Lett 378(2):98–101
Cunha RA, Ferré S, Vaugeois JM, Chen JF (2008) Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des 14(15):1512–1524
Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casadó V, Hillion J, Torvinen M et al (2004) Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat Disord 10(5):265–271
Fuxe K, Ferré S, Canals M, Torvinen M, Terasmaa A, Marcellino D, Goldberg SR, Staines W et al (2005) Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J Mol Neurosci 26(2–3):209–220
Wardas J (2008) Potential role of adenosine A2A receptors in the treatment of schizophrenia. Front Biosci 13:4071–4096
Heffner TG, Wiley JN, Williams AE, Bruns RF, Coughenour LL, Downs DA (1989) Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacology 98(1):31–37
Kafka SH, Corbett R (1996) Selective adenosine A2A receptor/dopamine D2 receptor interactions in animal models of schizophrenia. Eur J Pharmacol 295(2–3):147–154
Ferré S (1997) Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology (Berl.). 133(2):107–120
Lucas PB, Pickar D, Kelsoe J, Rapaport M, Pato C, Hommer D (1990) Effects of the acute administration of caffeine in patients with schizophrenia. Biol Psychiatry 28(1):35–40
Zaslove MO, Russell RL, Ross E (1991) Effect of caffeine intake on psychotic in-patients. Br J Psychiatry 159:565–567
Akhondzadeh S, Shasavand E, Jamilian H, Shabestari O, Kamalipour A (2000) Dipyridamole in the treatment of schizophrenia: adenosine-dopamine receptor interactions. J Clin Pharm Ther 25(2):131–137
MatuteC MM, Vallejo-Illarramendi A, Conti F (2005) Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia 49:451–455
Matos M, Shen H-Y, Augusto E, Wang Y, Wei CJ, Wang YT, Agostinho P, Boison D et al (2015) Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol Psychiatry 78:763–774
Domenici MR, Ferrante A, Martire A, Chiodi V, Pepponi R, Tebano MT, Popoli P (2019) Adenosine A2A receptor as potential therapeutic target in neuropsychiatric disorders. Pharmacol Res 147:104338
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955. [CrossRef]
Talbott EO, Malek AM, Lacomis D (2016) The epidemiology of amyotrophic lateral sclerosis. Handb Clin Neurol 138:225–238
Mori A, Cross B, Uchida S, Walker JK, Ristuccia R (2021) How are adenosine and adenosine A2A receptors involved in the pathophysiology of amyotrophic lateral sclerosis? Biomedicines 9:1027
de Lera RM, Lim Y-H, Zheng J (2014) Adenosine A2A receptor as a drug discovery target. J Med Chem 57(9):3623–3650
Fresco P, Diniz C, Gonçalves J (2004) Facilitation of noradrenaline release by activation of adenosine A(2A) receptors triggers both phospholipase C and adenylate cyclase pathways in rat tail artery. Cardiovasc Res 63(4):739–746
Fredholm BB, Chern Y, Franco R, Sitkovsky M (2007) Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol 83(5):263–276
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16(3):177–192
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K et al (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325(6106):733–736
Calabrò M, Rinaldi C, Santoro G, Crisafulli C (2020) The biological pathways of Alzheimer disease: a review. AIMS Neuroscience 8(1):86–132
Qi Y, Klyubin I, Harney SC et al (2014) Longitudinal testing of hippocampal plasticity reveals the onset and maintenance of endogenous human Ass-induced synaptic dysfunction in individual freely behaving pre-plaque transgenic rats: rapid reversal by anti-Ass agents. Acta NeuropatholCommun 2:175
Lo AC, Iscru E, Blum D et al (2013) Amyloid and tau neuropathology differentially affect prefrontal synaptic plasticity and cognitive performance in mouse models of Alzheimer’s disease. J Alzheimers Dis 37:109–125
Giulian D, Baker TJ (1986) Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci 6:2163–2178
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Mecha M, Carrillo-Salinas FJ, Feliu A et al (2016) Microglia activation states and cannabinoid system: therapeutic implications. PharmacolTher 166:40–55
Michaud JP, Hallé M, Lampron A, Thériault P, Préfontaine P, Filali M, Triboutjover P, Lanteigne AM et al (2013) Toll like receptor 4 stimulation with the detoxifed ligand mono-phosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc Natl Acad Sci USA 110:1941–1946
Tato PD, Holtman IR, Boddeke EW, Möller T, Eggen BJ (2016) Next generation transcriptomics and genomics elucidate biological complexity of microglia in health and disease. Glia 64(2):197–213
Henstridge CM, Hyman BT, Spires-Jones TL (2019) Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neuroscience 20(2):94–108
Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A et al (2013) CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neuroscience 16(7):848–850
Dai SS, Zhou YG (2011) Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Rev Neurosci 22:231–239
Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV et al (2007) Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol 83:310–331
Illes P, Rubini P, Ulrich H, Zhao Y, Tang Y (2020) Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells 9:1108
Merighi S, Borea PA, Varani K, Vincenzi F, Travagli A et al (2022) Pathophysiological role and medicinal chemistry of A2A adenosine receptor antagonists in Alzheimer’s disease. Molecules 27:2680
Saini A, Patel R, Gaba S, Gurpreet Singh GD, Gupta VikramdeepMonga (2022) Adenosine receptor antagonists: recent advances and therapeutic perspective. Eur J Med Chem 227:113907
Zheng J, Zhang X, Zhen X (2018) Development of adenosine A2A receptor antagonists for the treatment of Parkinson’s disease: a recent update and challenge. ACS Chem Neurosci 10(2):783–791
Poleszak E, Szopa A, Bogatko K et al (2019) Antidepressant-like activity of typical antidepressant drugs in the forced swim test and tail suspension test in mice is augmented by DMPX, an adenosine A 2A receptor antagonist. Neurotox Res 35(2):344–352
Acknowledgements
Dipanjan Karati is thankful to School of Pharmacy, Techno India University, for their continuous support.
Author information
Authors and Affiliations
Contributions
We declare that this work was done by the authors named in this article: SR and SWM conceived and designed the study. DK wrote the paper. SR and SWM drafted the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karati, D., Mukherjee, S. & Roy, S. Molecular and Structural Insight into Adenosine A2A Receptor in Neurodegenerative Disorders: A Significant Target for Efficient Treatment Approach. Mol Neurobiol 60, 5987–6000 (2023). https://doi.org/10.1007/s12035-023-03441-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03441-5