Abstract
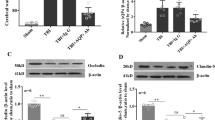
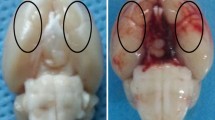
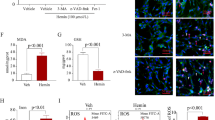
Decompressive craniectomy (DC) is a major form of surgery that is used to reduce intracranial hypertension (IH), the most frequent cause of death and disability following severe traumatic brain injury (sTBI) and stroke. Our previous research showed that controlled decompression (CDC) was more effective than rapid decompression (RDC) with regard to reducing the incidence of complications and improving outcomes after sTBI; however, the specific mechanisms involved have yet to be elucidated. In the present study, we investigated the effects of CDC in regulating inflammation after IH and attempted to identify the mechanisms involved. Analysis showed that CDC was more effective than RDC in alleviating motor dysfunction and neuronal death in a rat model of traumatic intracranial hypertension (TIH) created by epidural balloon pressurization. Moreover, RDC induced M1 microglia polarization and the release of pro-inflammatory cytokines. However, CDC treatment resulted in microglia primarily polarizing into the M2 phenotype and induced the significant release of anti-inflammatory cytokines. Mechanistically, the establishment of the TIH model led to the increased expression of hypoxia-inducible factor-1α (HIF-1α); CDC ameliorated cerebral hypoxia and reduced the expression of HIF-1α. In addition, 2-methoxyestradiol (2-ME2), a specific inhibitor of HIF-1α, significantly attenuated RDC-induced inflammation and improved motor function by promoting M1 to M2 phenotype transformation in microglial and enhancing the release of anti-inflammatory cytokines. However, dimethyloxaloylglycine (DMOG), an agonist of HIF-1α, abrogated the protective effects of CDC treatment by suppressing M2 microglia polarization and the release of anti-inflammatory cytokines. Collectively, our results indicated that CDC effectively alleviated IH-induced inflammation, neuronal death, and motor dysfunction by regulating HIF-1α-mediated microglial phenotype polarization. Our findings provide a better understanding of the mechanisms that underlie the protective effects of CDC and promote clinical translational research for HIF-1α in IH.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM et al (2016) Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 375(12):1119–1130. https://doi.org/10.1056/NEJMoa1605215
Tadevosyan A, Kornbluth J (2021) Brain herniation and intracranial hypertension. Neurologic Clinics 39(2):293–318. https://doi.org/10.1016/j.ncl.2021.02.005
Robba C, Graziano F, Rebora P, Elli F, Giussani C, Oddo M, Meyfroidt G, Helbok R, Taccone FS, Prisco L, Vincent J-L, Suarez JI, Stocchetti N, Citerio G (2021) Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): an international, prospective observational cohort study. Lancet Neurol 20(7):548–558. https://doi.org/10.1016/S1474-4422(21)00138-1
Smith M (2017) Refractory intracranial hypertension: the role of decompressive craniectomy. Anesth Analg 125(6):1999–2008. https://doi.org/10.1213/ANE.0000000000002399
Chen J, Li M, Chen L, Chen W, Zhang C, Feng Y, Wang Y, Chen Q (2020) The effect of controlled decompression for severe traumatic brain injury: a randomized, controlled trial. Front Neurol 11:107. https://doi.org/10.3389/fneur.2020.00107
Chen T, Qian X, Zhu J, Yang L-K, Wang Y-H (2021) Controlled decompression attenuates compressive injury following traumatic brain injury via TREK-1-mediated inhibition of necroptosis and neuroinflammation. Oxid Med Cell Longev 2021:4280951. https://doi.org/10.1155/2021/4280951
Qian X, Zhang C, Zhou Z, Cao X, Zhang C, Chen T, Wang Y (2022) Controlled decompression attenuates brain damage in a rat model of epidural extreme intracranial hypertension: Partially via inhibiting necroptosis and inflammatory response. Neurochem Int 153:105257. https://doi.org/10.1016/j.neuint.2021.105257
Zhang C, Qian X, Zheng J, Ai P, Cao X, Pan X, Chen T, Wang Y (2022) Controlled decompression alleviates brain injury via attenuating oxidative damage and neuroinflammation in acute intracranial hypertension. Biomed Res Int 2022:1936691. https://doi.org/10.1155/2022/1936691
Jia Y, Wang G, Ye Y, Kang E, Chen H, Guo Z, He X (2021) Niche cells crosstalk in neuroinflammation after traumatic brain injury. Int J Biol Sci 17(1):368–378. https://doi.org/10.7150/ijbs.52169
Candelario-Jalil E, Dijkhuizen RM, Magnus T (2022) Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke 53(5):1473–1486. https://doi.org/10.1161/STROKEAHA.122.036946
Voet S, Prinz M, van Loo G (2019) Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med 25(2):112–123. https://doi.org/10.1016/j.molmed.2018.11.005
Watts ER, Walmsley SR (2019) Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol Med 25(1):33–46. https://doi.org/10.1016/j.molmed.2018.10.006
Liu R, Liao X-Y, Pan M-X, Tang J-C, Chen S-F, Zhang Y, Lu P-X, Lu LJ, Zou Y-Y, Qin X-P, Bu L-H, Wan Q (2019) Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J Immunol 202(6):1704–1714. https://doi.org/10.4049/jimmunol.1801166
Ferraro E, Germanò M, Mollace R, Mollace V, Malara N (2021) HIF-1, the Warburg Effect, and macrophage/microglia polarization potential role in COVID-19 pathogenesis. Oxid Med Cell Longev 2021:8841911. https://doi.org/10.1155/2021/8841911
Gao J, Yao M, Zhang W, Yang B, Yuan G, Liu J-X, Zhang Y (2022) Panax notoginseng saponins alleviates inflammation induced by microglial activation and protects against ischemic brain injury via inhibiting HIF-1α/PKM2/STAT3 signaling. Biomed Pharmacother 155:113479. https://doi.org/10.1016/j.biopha.2022.113479
Kim S-R, Seong K-J, Kim W-J, Jung J-Y (2022) Epigallocatechin gallate protects against hypoxia-induced inflammation in microglia via NF-κB suppression and Nrf-2/HO-1 activation. Int J Mol Sci 23:7. https://doi.org/10.3390/ijms23074004
Fang Y, Lu J, Wang X, Wu H, Mei S, Zheng J, Xu S, Lenahan C, Chen S, Zhang J, Hong Y (2020) HIF-1α mediates TRAIL-induced neuronal apoptosis regulating DcR1 expression following traumatic brain injury. Front Cell Neurosci 14:192. https://doi.org/10.3389/fncel.2020.00192
Yang Y, Chen X, Feng Z, Cai X, Zhu X, Cao M, Yang L, Chen Y, Wang Y, Feng H (2022) MEC17-induced α-tubulin acetylation restores mitochondrial transport function and alleviates axonal injury after intracerebral hemorrhage in mice. Journal of neurochemistry 160(1):51–63. https://doi.org/10.1111/jnc.15493
Schaible E-V, Windschügl J, Bobkiewicz W, Kaburov Y, Dangel L, Krämer T, Huang C, Sebastiani A, Luh C, Werner C, Engelhard K, Thal SC, Schäfer MKE (2014) 2-Methoxyestradiol confers neuroprotection and inhibits a maladaptive HIF-1α response after traumatic brain injury in mice. J Neurochem 129(6):940–954. https://doi.org/10.1111/jnc.12708
Xiong A, Li J, Xiong R, Xia Y, Jiang X, Cao F, Lu H, Xu J, Shan F (2022) Inhibition of HIF-1α-AQP4 axis ameliorates brain edema and neurological functional deficits in a rat controlled cortical injury (CCI) model. Sci Rep 12(1):2701. https://doi.org/10.1038/s41598-022-06773-9
Gao Z, Pang Z, Lei G, Chen Y, Cai Z, Zhu S, Lin W, Qiu Z, Wang Y, Shen Y, Xu W (2022) Crossing nerve transfer drives sensory input-dependent plasticity for motor recovery after brain injury. Sci Adv 8(35):eabn5899. https://doi.org/10.1126/sciadv.abn5899
Metz GA, Whishaw IQ (2009) The ladder rung walking task: a scoring system and its practical application. J Vis Exp 28:e1204. https://doi.org/10.3791/1204
Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M (2001) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32(11):2682–2688
Chen W-K, Feng L-J, Liu Q-D, Ke Q-F, Cai P-Y, Zhang P-R, Cai L-Q, Huang N-L, Lin W-P (2020) Inhibition of leucine-rich repeats and calponin homology domain containing 1 accelerates microglia-mediated neuroinflammation in a rat traumatic spinal cord injury model. J Neuroinflammation 17(1):202. https://doi.org/10.1186/s12974-020-01884-4
Kim S, Son Y (2021) Astrocytes stimulate microglial proliferation and M2 polarization in vitro through crosstalk between astrocytes and microglia. Int J Mol Sci 22:16. https://doi.org/10.3390/ijms22168800
Lafrenaye AD, Krahe TE, Povlishock JT (2014) Moderately elevated intracranial pressure after diffuse traumatic brain injury is associated with exacerbated neuronal pathology and behavioral morbidity in the rat. J Cereb Blood Flow Metab 34(10):1628–1636. https://doi.org/10.1038/jcbfm.2014.122
Zhu X, Hao W, Liu Z, Song Y, Hao C, Wu S, Lu X, Yang J, Jin C (2022) Aluminum induces neuroinflammation via P2X7 receptor activating NLRP3 inflammasome pathway. Ecotoxicol Environ Saf 249:114373. https://doi.org/10.1016/j.ecoenv.2022.114373
Xu S, Wang J, Zhong J, Shao M, Jiang J, Song J, Zhu W, Zhang F, Xu H, Xu G, Zhang Y, Ma X, Lyu F (2021) CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin Transl Med 11(1):e269. https://doi.org/10.1002/ctm2.269
Wei Y, Chen J, Cai G-E, Lu W, Xu W, Wang R, Lin Y, Yang C (2021) Rosmarinic acid regulates microglial M1/M2 polarization via the PDPK1/Akt/HIF pathway under conditions of neuroinflammation. Inflammation 44(1):129–147. https://doi.org/10.1007/s10753-020-01314-w
Chen Z-Q, Yu H, Li H-Y, Shen H-T, Li X, Zhang J-Y, Zhang Z-W, Wang Z, Chen G (2019) Negative regulation of glial Tim-3 inhibits the secretion of inflammatory factors and modulates microglia to antiinflammatory phenotype after experimental intracerebral hemorrhage in rats. CNS Neurosci Ther 25(6):674–684. https://doi.org/10.1111/cns.13100
Canac N, Jalaleddini K, Thorpe SG, Thibeault CM, Hamilton RB (2020) Review: pathophysiology of intracranial hypertension and noninvasive intracranial pressure monitoring. Fluids Barriers CNS 17(1):40. https://doi.org/10.1186/s12987-020-00201-8
Changa AR, Czeisler BM, Lord AS (2019) Management of elevated intracranial pressure: a review. Curr Neurol Neurosci Rep 19(12):99. https://doi.org/10.1007/s11910-019-1010-3
Hawryluk GWJ, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, Arrastia RD, Diringer M, Figaji A, Gao G, Geocadin R, Ghajar J, Harris O, Hoffer A, Hutchinson P, Joseph M, Kitagawa R, Manley G, Mayer S et al (2019) A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 45(12):1783–1794. https://doi.org/10.1007/s00134-019-05805-9
Honeybul S, Ho KM, Lind CRP, Gillett GR (2010) Observed versus predicted outcome for decompressive craniectomy: a population-based study. J Neurotrauma 27(7):1225–1232. https://doi.org/10.1089/neu.2010.1316
Sahuquillo J, Dennis JA (2019) Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev 12:CD003983. https://doi.org/10.1002/14651858.CD003983.pub3
Hawryluk GWJ, Rubiano AM, Totten AM, O'Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Lumba-Brown A, Ghajar J (2020) Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery 87(3):427–434. https://doi.org/10.1093/neuros/nyaa278
Nagai M, Ishikawa M, Matsumoto E, Arai F, Oguma H, Hashimoto M (2020) Exploration of an Easy and simple method for decompressive craniectomy: the “spiral dural incision method”. Neurol Med Chir (Tokyo) 60(9):475–481. https://doi.org/10.2176/nmc.cr.2019-0289
Alam HB, Vercruysse G, Martin M, Brown CVR, Brasel K, Moore EE, Sava J, Ciesla D, Inaba K (2020) Western Trauma association critical decisions in trauma: management of intracranial hypertension in patients with severe traumatic brain injuries. J Trauma Acute Care Surg 88(2):345–351. https://doi.org/10.1097/TA.0000000000002555
Chen H, Ma D, Yue F, Qi Y, Dou M, Cui L, Xing Y (2022) The potential role of hypoxia-inducible factor-1 in the progression and therapy of central nervous system diseases. Curr Neuropharmacol 20(9):1651–1666. https://doi.org/10.2174/1570159X19666210729123137
Yuan D, Guan S, Wang Z, Ni H, Ding D, Xu W, Li G (2021) HIF-1α aggravated traumatic brain injury by NLRP3 inflammasome-mediated pyroptosis and activation of microglia. J Chem Neuroanat 116:101994. https://doi.org/10.1016/j.jchemneu.2021.101994
Du X, Yang J, Liu C, Wang S, Zhang C, Zhao H, Du H, Geng X (2020) Hypoxia-inducible factor 1α and 2α have beneficial effects in remote ischemic preconditioning against stroke by modulating inflammatory responses in aged rats. Front Aging Neurosci 12:54. https://doi.org/10.3389/fnagi.2020.00054
Galván-Peña S, O'Neill LAJ (2014) Metabolic reprograming in macrophage polarization. Front Immunol 5:420. https://doi.org/10.3389/fimmu.2014.00420
de Lemos ML, de la Torre AV, Petrov D, Brox S, Folch J, Pallàs M, Lazarowski A, Beas-Zarate C, Auladell C, Camins A (2013) Evaluation of hypoxia inducible factor expression in inflammatory and neurodegenerative brain models. Int J Biochem Cell Biol 45(7):1377–1388. https://doi.org/10.1016/j.biocel.2013.04.011
Fang H-Y, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, Johnson RS, Imityaz HZ, Simon MC, Fredlund E, Greten FR, Rius J, Lewis CE (2009) Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood 114(4):844–859. https://doi.org/10.1182/blood-2008-12-195941
He Q, Ma Y, Liu J, Zhang D, Ren J, Zhao R, Chang J, Guo Z-N, Yang Y (2021) Biological functions and regulatory mechanisms of hypoxia-inducible factor-1α in ischemic stroke. Front Immunol 12:801985. https://doi.org/10.3389/fimmu.2021.801985
Zhao Y, Wei ZZ, Lee JH, Gu X, Sun J, Dix TA, Wei L, Yu SP (2020) Pharmacological hypothermia induced neurovascular protection after severe stroke of transient middle cerebral artery occlusion in mice. Exp Neurol 325:113133. https://doi.org/10.1016/j.expneurol.2019.113133
Xu X, Yin D, Ren H, Gao W, Li F, Sun D, Wu Y, Zhou S, Lyu L, Yang M, Xiong J, Han L, Jiang R, Zhang J (2018) Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol Dis 117:15–27. https://doi.org/10.1016/j.nbd.2018.05.016
Bae Y-H, Joo H, Bae J, Hyeon SJ, Her S, Ko E, Choi HG, Ryu H, Hur E-M, Bu Y, Lee BD (2018) Brain injury induces HIF-1α-dependent transcriptional activation of LRRK2 that exacerbates brain damage. Cell Death Dis 9(11):1125. https://doi.org/10.1038/s41419-018-1180-y
Hubbard WB, Dong J-F, Cruz MA, Rumbaut RE (2021) Links between thrombosis and inflammation in traumatic brain injury. Thromb Res 198:62–71. https://doi.org/10.1016/j.thromres.2020.10.041
Zhao D, Zhang LJ, Huang TQ, Kim J, Gu M-Y, Yang HO (2021) Narciclasine inhibits LPS-induced neuroinflammation by modulating the Akt/IKK/NF-κB and JNK signaling pathways. Phytomedicine 85:153540. https://doi.org/10.1016/j.phymed.2021.153540
Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, Fu H, Li Y (2018) Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation 15(1):116. https://doi.org/10.1186/s12974-018-1151-3
Chitnis T, Weiner HL (2017) CNS inflammation and neurodegeneration. J Clin Invest 127(10):3577–3587. https://doi.org/10.1172/JCI90609
Le Thuc O, Blondeau N, Nahon J-L, Rovère C (2015) The complex contribution of chemokines to neuroinflammation: switching from beneficial to detrimental effects. Ann N Y Acad Sci 1351:127–140. https://doi.org/10.1111/nyas.12855
Qin C, Zhou L-Q, Ma X-T, Hu Z-W, Yang S, Chen M, Bosco DB, Wu L-J, Tian D-S (2019) Dual functions of microglia in ischemic stroke. Neurosci Bull 35(5):921–933. https://doi.org/10.1007/s12264-019-00388-3
Ma Y, Wang J, Wang Y, Yang G-Y (2017) The biphasic function of microglia in ischemic stroke. Prog Neurobiol 157:247–272. https://doi.org/10.1016/j.pneurobio.2016.01.005
Shi M, Mi L, Li F, Li Y, Zhou Y, Chen F, Liu L, Chai Y, Yang W, Zhang J, Chen X (2022) Fluvoxamine confers neuroprotection via inhibiting infiltration of peripheral leukocytes and M1 polarization of microglia/macrophages in a mouse model of traumatic brain injury. J Neurotrauma 9(17-18):1240–1261. https://doi.org/10.1089/neu.2021.0355
Long X, Yao X, Jiang Q, Yang Y, He X, Tian W, Zhao K, Zhang H (2020) Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation 17(1):89. https://doi.org/10.1186/s12974-020-01761-0
Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J (2017) Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 95(6):1246–1265. https://doi.org/10.1016/j.neuron.2017.07.010
Goshi N, Morgan RK, Lein PJ, Seker E (2020) A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J Neuroinflammation 17(1):155. https://doi.org/10.1186/s12974-020-01819-z
Sacristán C (2020) Microglia and astrocyte crosstalk in immunity. Trends Immunol 41(9):747–748. https://doi.org/10.1016/j.it.2020.07.009
Cherry JD, Olschowka JA, O'Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. https://doi.org/10.1186/1742-2094-11-98
Sica A, Erreni M, Allavena P, Porta C (2015) Macrophage polarization in pathology. Cell Mol Life Sci 72(21):4111–4126. https://doi.org/10.1007/s00018-015-1995-y
Jordão MJC, Sankowski R, Brendecke SM, Sagar LG, Tai Y-H, Tay TL, Schramm E, Armbruster S, Hagemeyer N, Groß O, Mai D, Çiçek Ö, Falk T, Kerschensteiner M, Grün D, Prinz M (2019) Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363:6425. https://doi.org/10.1126/science.aat7554
Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M (2016) Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr Neuropharmacol 14(6):641–653
Han J, Fan Y, Zhou K, Blomgren K, Harris RA (2021) Uncovering sex differences of rodent microglia. J Neuroinflammation 18(1):74. https://doi.org/10.1186/s12974-021-02124-z
Kerr N, Dietrich DW, Bramlett HM, Raval AP (2019) Sexually dimorphic microglia and ischemic stroke. CNS Neurosci Ther 25(12):1308–1317. https://doi.org/10.1111/cns.13267
Funding
The current study was funded by the National Natural Science Foundation of China (No.81871589), the Military Logistics Scientific Research Project (No. CLB20J027), Wuxi Municipal Bureau on Science and Technology (N20202037), the Key scientific research project of Jiangsu Provincial Health Commission (K2019018), the Wuxi Municipal Social Development Science and Technology Demonstration Project (N20201008), the Natural Science Foundation of Jiangsu Province (BK20221206), and the Young Elite Scientists Sponsorship Program of Jiangsu Province (TJ-2022-028).
Author information
Authors and Affiliations
Contributions
YHW, LKY, and JZ contributed to the conception and design of the study. JZ, YY, CXZ, YHW, CHZ, YYC, and WZ acquired and analyzed the data. JZ and YHW drafted a significant portion of the manuscript or figures. All authors read and approved the present version of the manuscript to be published.
Corresponding authors
Ethics declarations
Ethics Approval
All experiments are reported in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. The experimental protocols were approved by the Laboratory Animal Welfare and Ethics Committee of the 904th Hospital of PLA (20220215) and performed according to the Guide for the Care and Use of Laboratory Animals.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
All authors have seen and approved the manuscript and contributed significantly to this work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Zheng, Chenxu Zhang, and Yonghui Wu contributed equally to this manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, J., Zhang, C., Wu, Y. et al. Controlled Decompression Alleviates Motor Dysfunction by Regulating Microglial Polarization via the HIF-1α Signaling Pathway in Intracranial Hypertension. Mol Neurobiol 60, 5607–5623 (2023). https://doi.org/10.1007/s12035-023-03416-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03416-6