Abstract
Optimizing the metabolic phenotype to improve cerebral function is critical for treatment of cerebral ischemia-reperfusion (I/R) injury. Guhong injection (GHI), which comprised safflower extract and aceglutamide, is widely prescribed in Chinese medicine for the treatment of cerebrovascular diseases. In this study, a combination of LC-QQQ-MS and MALDI–MSI were utilized to explore tissue-specific metabolic alterations in the brain of I/R, as well as to evaluate the therapeutic effect of GHI. Pharmacological evaluation demonstrated that GHI can significantly improve infarction rate, neurological deficit, cerebral blood flow, and neuronal damage in I/R rats. Based on LC-QQQ-MS, 23 energy metabolites were found to be significantly altered in the I/R group compared to the sham group (P < 0.05). After GHI treatment, 12 metabolites, including G6P, TPP, NAD, citrate, succinate, malate, ATP, GTP, GDP, ADP, NADP, and FMN showed a significant tendency of returning to baseline values (P < 0.05). Based on MALDI-MSI, 4 metabolites in glycolysis and TCA, 4 metabolites in nucleic acid metabolism, 4 amino acid metabolites, and 6 metabolites were discovered and compared between the different groups in the four special regions of cortex, hippocampus, hypothalamus, and striatum. Parts of these were found to have significant changes after I/R in the special brain region, and were regulated by GHI. The study provides comprehensive and detailed information for specific metabolic reprogramming of brain tissue in rats with I/R, and the therapeutic effect of GHI.
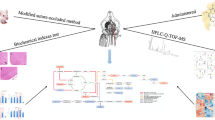
Graphical Abstract
Schema describing the discovery strategies of integrated LC-MS and MALDI-MSI to identify cerebral ischemia reperfusion metabolic reprogramming and GHI therapeutic effects








Similar content being viewed by others
Data Availability
The datasets used and/or analyzed in the current study are available from the corresponding authors on reasonable request.
Abbreviations
- ADP:
-
Adenosine diphosphate
- ATP:
-
Denosine triphosphate
- BBB:
-
Blood brain barrier;
- CI:
-
Cerebral ischemia
- GHI:
-
Guhong injection
- GC–MS:
-
Chromatography-mass spectrometry
- GDP:
-
Guanosine diphosphate
- LC-MS/MS:
-
Liquid chromatography/tandem mass spectrometry
- LysoPC:
-
Lysophosphatidylcholine
- MALDI–MSI:
-
Matrix-assisted laser desorption ionization mass spectrometry imaging
- NMR:
-
Nuclear magnetic resonance
- PC:
-
Phosphatidylcholine
- PI:
-
Phosphatidylinositol
- PA:
-
Phosphatidic acid
- tPA:
-
Tissue plasminogen activator
- TPP:
-
Thiamine pyrophosphate succinate
References
Zhang X, Wei M, Fan J et al (2021) Ischemia-induced upregulation of autophagy preludes dysfunctional lysosomal storage and associated synaptic impairments in neurons. Autophagy 17:1519–1542. https://doi.org/10.1080/15548627.2020.1840796
Zhou Z, Lu J, Liu WW et al (2018) Advances in stroke pharmacology. Pharmacol Ther 191:23–42. https://doi.org/10.1016/j.pharmthera.2018.05.012
Gao X, Yang H, Su J et al (2020) Aescin protects neuron from ischemia-reperfusion injury via regulating the PRAS40/mTOR signaling pathway. Oxid Med Cell Longev 2020:7815325. https://doi.org/10.1155/2020/7815325
Jangholi E, Sharifi ZN, Hoseinian M et al (2020) Verapamil inhibits mitochondria-induced reactive oxygen species and dependent apoptosis pathways in cerebral transient global ischemia/reperfusion. Oxid Med Cell Longev 2020:5872645. https://doi.org/10.1155/2020/5872645
Wardlaw JM, Murray V, Berge E, del Zoppo GJ (2014) Thrombolysis for acute ischaemic stroke. The. Cochrane Database Syst Rev 2014:Cd000213. https://doi.org/10.1002/14651858.CD000213.pub3
Vidale S, Agostoni E (2014) Thrombolysis in acute ischaemic stroke. Brain 137:e281–e281. https://doi.org/10.1093/brain/awu065
Liu M, Xu Z, Wang L et al (2020) Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte. J Neuroinflammation 17:270. https://doi.org/10.1186/s12974-020-01946-7
Xing C, Arai K, Lo EH, Hommel M (2012) Pathophysiologic cascades in ischemic stroke. Int J Stroke 7:378–385. https://doi.org/10.1111/j.1747-4949.2012.00839.x
Isaev NK, Stelmashook EV, Plotnikov EY et al (2008) Role of acidosis, NMDA receptors, and acid-sensitive ion channel 1a (ASIC1a) in neuronal death induced by ischemia. Biochemistry (Mosc) 73:1171–1175. https://doi.org/10.1134/s0006297908110011
Dong XX, Wang Y, Qin ZH (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30:379–387. https://doi.org/10.1038/aps.2009.24
Jiang X, Andjelkovic AV, Zhu L et al (2018) Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 163–164:144–171. https://doi.org/10.1016/j.pneurobio.2017.10.001
Liu F, Lu J, Manaenko A et al (2018) Mitochondria in ischemic stroke: new insight and implications. Aging Dis 9:924–937. https://doi.org/10.14336/AD.2017.1126
Vidale S, Consoli A, Arnaboldi M, Consoli D (2017) Postischemic inflammation in acute stroke. J Clin Neurol 13:1–9. https://doi.org/10.3988/jcn.2017.13.1.1
Huang Q, Yin P, Wang J et al (2011) Method for liver tissue metabolic profiling study and its application in type 2 diabetic rats based on ultra performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879:961–967. https://doi.org/10.1016/j.jchromb.2011.03.009
Huang Q, Tan Y, Yin P et al (2013) Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res 73:4992–5002. https://doi.org/10.1158/0008-5472.CAN-13-0308
van der Greef J, Leegwater DC (1983) Urine profile analysis by field desorption mass spectrometry, a technique for detecting metabolites of xenobiotics. Application to 3,5-dinitro-2-hydroxytoluene. Biomed Mass Spectrom 10:1–4. https://doi.org/10.1002/bms.1200100102
Pérez-Trujillo M, Athersuch TJ (2021) Special Issue: NMR-based metabolomics. Molecules (Basel, Switzerland) 26. https://doi.org/10.3390/molecules26113283
Shariatgorji M, Nilsson A, Fridjonsdottir E et al (2019) Comprehensive mapping of neurotransmitter networks by MALDI-MS imaging. Nat Methods 16:1021–1028. https://doi.org/10.1038/s41592-019-0551-3
Sun C, Li T, Song X et al (2019) Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proc Natl Acad Sci U S A 116:52–57. https://doi.org/10.1073/pnas.1808950116
He J, Sun C, Li T et al (2018) A sensitive and wide coverage ambient mass spectrometry imaging method for functional metabolites based molecular histology. Adv Sci (Weinh) 5:1800250. https://doi.org/10.1002/advs.201800250
Eveque-Mourroux MR, Emans PJ, Zautsen RRM et al (2019) Spatially resolved endogenous improved metabolite detection in human osteoarthritis cartilage by matrix assisted laser desorption ionization mass spectrometry imaging. Analyst 144:5953–5958. https://doi.org/10.1039/c9an00944b
Liu X, Flinders C, Mumenthaler SM, Hummon AB (2018) MALDI mass spectrometry imaging for evaluation of therapeutics in colorectal tumor organoids. J Am Soc Mass Spectrom 29:516–526. https://doi.org/10.1007/s13361-017-1851-4
Liu X, Hummon AB (2016) Chemical imaging of platinum-based drugs and their metabolites. Sci Rep 6:38507. https://doi.org/10.1038/srep38507
Dudley E (2019) MALDI Profiling and applications in medicine. Adv Exp Med Biol 1140:27–43. https://doi.org/10.1007/978-3-030-15950-4_2
Chaurand P, Norris JL, Cornett DS et al (2006) New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrometry. J Proteome Res 5:2889–2900. https://doi.org/10.1021/pr060346u
Ahmed M, Broeckx G, Baggerman G et al (2020) Next-generation protein analysis in the pathology department. J Clin Pathol 73:1–6. https://doi.org/10.1136/jclinpath-2019-205864
Andrews WT, Skube SB, Hummon AB (2017) Magnetic bead-based peptide extraction methodology for tissue imaging. Analyst 143:133–140. https://doi.org/10.1039/c7an00757d
Clemis EJ, Smith DS, Camenzind AG et al (2012) Quantitation of spatially-localized proteins in tissue samples using MALDI-MRM imaging. Anal Chem 84:3514–3522. https://doi.org/10.1021/ac202875d
Tobias F, Olson MT, Cologna SM (2018) Mass spectrometry imaging of lipids: untargeted consensus spectra reveal spatial distributions in Niemann-Pick disease type C1. J Lipid Res 59:2446–2455. https://doi.org/10.1194/jlr.D086090
Nielsen MM, Lambertsen KL, Clausen BH et al (2016) Mass spectrometry imaging of biomarker lipids for phagocytosis and signalling during focal cerebral ischaemia. Sci Rep 6:39571. https://doi.org/10.1038/srep39571
Mallah K, Quanico J, Raffo-Romero A et al (2019) Matrix-assisted laser desorption/ionization-mass spectrometry imaging of lipids in experimental model of traumatic brain injury detecting acylcarnitines as injury related markers. Anal Chem 91:11879–11887. https://doi.org/10.1021/acs.analchem.9b02633
Oppenheimer SR, Wehr AY (2015) Imaging mass spectrometry in drug discovery and development. Bioanalysis 7:2609–2610. https://doi.org/10.4155/bio.15.202
Ai J, Wan H, Shu M et al (2017) Guhong injection protects against focal cerebral ischemia-reperfusion injury via anti-inflammatory effects in rats. Arch Pharm Res 40:610–622. https://doi.org/10.1007/s12272-016-0835-4
Zhang J, Zhou R, Xiang C et al (2020) Enhanced thioredoxin, glutathione and Nrf2 antioxidant systems by safflower extract and aceglutamide attenuate cerebral ischaemia/reperfusion injury. J Cell Mol Med 24:4967–4980. https://doi.org/10.1111/jcmm.15099
Fan F, Chen S, Yang H et al (2018) The investigation of molecular mechanism of Guhong injection against cerebral ischemia-reperfusion injury in a network pharmacology approach[J]. Complex Systems and Complexity Science. https://doi.org/10.13306/j.1672-3813.2018.01.002
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91. https://doi.org/10.1161/01.str.20.1.84
Zhao J, Ouyang Y, Wang H et al (2022) An energy metabolism study on the efficacy of naoxintong capsules against myocardial infarction in a rat model. Oxid Med Cell Longev 2022:3712500. https://doi.org/10.1155/2022/3712500
Wahul AB, Joshi PC, Kumar A, Chakravarty S (2018) Transient global cerebral ischemia differentially affects cortex, striatum and hippocampus in Bilateral Common Carotid Arterial occlusion (BCCAo) mouse model. J Chem Neuroanat 92:1–15. https://doi.org/10.1016/j.jchemneu.2018.04.006
Frenguelli BG, Dale N (2020) Purines: from diagnostic biomarkers to therapeutic agents in brain injury. Neurosci Bull 36:1315–1326. https://doi.org/10.1007/s12264-020-00529-z
Irie M, Fujimura Y, Yamato M et al (2014) Integrated MALDI-MS imaging and LC-MS techniques for visualizing spatiotemporal metabolomic dynamics in a rat stroke model. Metabolomics 10:473–483. https://doi.org/10.1007/s11306-013-0588-8
Yousuf S, Atif F, Ahmad M et al (2009) Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res 1250:242–253. https://doi.org/10.1016/j.brainres.2008.10.068
Sarkar S, Das N (2006) Mannosylated liposomal flavonoid in combating age-related ischemia-reperfusion induced oxidative damage in rat brain. Mech Ageing Dev 127:391–397. https://doi.org/10.1016/j.mad.2005.12.010
Hertz L, Chen Y (2017) Integration between glycolysis and glutamate-glutamine cycle flux may explain preferential glycolytic increase during brain activation, Requiring Glutamate. Front Integr Neurosci 11:18. https://doi.org/10.3389/fnint.2017.00018
Kim AY, Jeong KH, Lee JH et al (2017) Glutamate dehydrogenase as a neuroprotective target against brain ischemia and reperfusion. Neuroscience 340:487–500. https://doi.org/10.1016/j.neuroscience.2016.11.007
Ferrari F, Gorini A, Hoyer S, Villa RF (2018) Glutamate metabolism in cerebral mitochondria after ischemia and post-ischemic recovery during aging: relationships with brain energy metabolism. J Neurochem 146:416–428. https://doi.org/10.1111/jnc.14464
Zhang LN, Hao L, Guo YS et al (2019) Are glutamate transporters neuroprotective or neurodegenerative during cerebral ischemia? J Mol Med (Berl) 97:281–289. https://doi.org/10.1007/s00109-019-01745-5
Mayor D, Tymianski M (2018) Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology 134:178–188. https://doi.org/10.1016/j.neuropharm.2017.11.050
Lin S, Zhou G, Shao W, Fu Z (2017) Impact of dexmedetomidine on amino acid contents and the cerebral ultrastructure of rats with cerebral ischemia-reperfusion injury. Acta Cir Bras 32:459–466. https://doi.org/10.1590/s0102-865020170060000006
Simao F, Matte A, Breier AC et al (2013) Resveratrol prevents global cerebral ischemia-induced decrease in lipid content. Neurol Res 35:59–64. https://doi.org/10.1179/1743132812Y.0000000116
Liu M, Tang L, Liu X et al (2016) An evidence-based review of related metabolites and metabolic network research on cerebral ischemia. Oxid Med Cell Longev 2016:9162074. https://doi.org/10.1155/2016/9162074
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568. https://doi.org/10.1152/physrev.1999.79.4.1431
Shanta SR, Choi CS, Lee JH et al (2012) Global changes in phospholipids identified by MALDI MS in rats with focal cerebral ischemia. J Lipid Res 53:1823–1831. https://doi.org/10.1194/jlr.M022558
Kubota M, Nakane M, Nakagomi T et al (2001) Regional distribution of ethanolamine plasmalogen in the hippocampal CA1 and CA3 regions and cerebral cortex of the gerbil. Neurosci Lett 301:175–178. https://doi.org/10.1016/s0304-3940(01)01631-7
Acknowledgements
We want to thank the participants for taking part in the study.
Funding
The research was supported by the National Key R&D Program of China (2019YFC1708900), National Natural Science Foundation of China (Nos. 81974550, 81973711), Scientific and Technological Innovation project of China Academy of Chinese Medical Science (CI2021A04612, CI2021A05208, CI2021B017), and The Fundamental Research Funds for the Central Public Welfare Research Institutes (JBGS2021003, ZZ13-YQ-080).
Author information
Authors and Affiliations
Contributions
Jingjing Zhang, Hongwei Wu, and Hongjun Yang conceived and designed the experiments; Huanhuan Wang, Guangzhao Cao, Rui Zhou, and Zhenkun Li performed the experiments; Caifeng Li, Liying Tang, and Xianyu Li analyzed the data; Huanhuan Wang and Hongwei Wu wrote the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All experimental animal procedures were approved by the China Academy of Chinese Medical Sciences’ Administrative Panel on Laboratory Animal Care and performed in accordance with institutional guidelines and ethics of the committee as part of the China Academy of Chinese Medical Sciences (code, ERCCACMS21-2106-17).
Consent for Publication
Not applicable.
Research Involving Human Participants and/or Animals
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Li, Z., Cao, G. et al. Targeted Energy Metabolomics Combined with Spatial Metabolomics Study on the Efficacy of Guhong Injection Against Cerebral Ischemia Reperfusion. Mol Neurobiol 60, 5533–5547 (2023). https://doi.org/10.1007/s12035-023-03403-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03403-x