Abstract
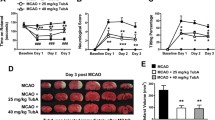
Cerebral ischemic stroke is one of the leading causes of human death. The fibrous scar is one of major factors influencing repair in central nervous system (CNS) injury. Silencing information regulator 2-related enzyme 1 (Sirt1) can regulate peripheral tissue and organ fibrosis. However, it is unclear how the fibrous scar forms and is regulated and it is unknown whether and how Sirt1 regulates the formation of the fibrous scar after cerebral ischemic stroke. Therefore, in the present study, we examined the effects of Sirt1 on the formation of the fibrotic scar after middle cerebral artery occlusion/reperfusion (MCAO/R) injury in vivo and on the transforming growth factor β1 (TGF-β1)-induced meningeal fibroblast fibrotic response in vitro, and we explored the molecular mechanisms underlying the Sirt1-regulated fibrosis process in vitro. We found that MCAO/R injury induced fibrotic scar formation in the ischemic area, which was accompanied by the downregulation of Sirt1 expression. The overexpression of Sirt1 reduced the infarct volume, improved Nissl body structure and reduced neurons injury, attenuated formation of fibrotic scar, upregulated growth associated protein43 (GAP43) and synaptophysin (SYP) expression, and promoted neurological function recovery. Similarly, Sirt1 expression was also downregulated in the TGF-β1-induced fibrosis model. Sirt1 overexpression inhibited fibroblast migration, proliferation, transdifferentiation into myofibroblasts, and secretion of extracellular matrix(ECM) by regulating the deacetylation of lysine at K49 and K120 sites of 14–3-3ζ in vitro. Therefore, we believe that Sirt1 could regulate fibrous scar formation and improve neurological function after cerebral ischemic stroke through regulating deacetylation of 14–3-3ζ.
Graphical Abstract









Similar content being viewed by others
Data Availability
All data are available upon reasonable request.
References
Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021. 20(10): 795–820.
Lee R, Lee M, Wu C et al (2018) Cerebral ischemia and neuroregeneration. Neural Regen Res 13(3):373–385
Rabinstein AA (2020) Update on treatment of acute ischemic stroke. Continuum 26(2):268–286
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453(7193):314–321
Fernández-Klett F, Priller J (2014) The fibrotic scar in neurological disorders. Brain Pathol 24(4):404–413
Dias DO, Göritz C (2018) Fibrotic scarring following lesions to the central nervous system. Matrix Biol 68–69:561–570
Dorrier CE, Aran D, Haenelt EA et al (2021) CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat Neurosci 24(2):234–244
Kawano H, Kimura-Kuroda J, Komuta Y et al (2012) Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res 349(1):169–180
Michan S, Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404(1):1–13
Huang XZ, Wen D, Zhang M et al (2014) Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J Cell Biochem 115(5):996–1005
Zhang Y, Connelly KA, Thai K et al (2017) Sirtuin 1 activation reduces transforming growth factor-β1-induced fibrogenesis and affords organ protection in a model of progressive, experimental kidney and associated cardiac disease. Am J Pathol 187(1):80–90
Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L (2013) SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep 3(4):1175–1186
Bugyei-Twum A, Ford C, Civitarese R et al (2018) Sirtuin 1 activation attenuates cardiac fibrosis in a rodent pressure overload model by modifying Smad2/3 transactivation. Cardiovasc Res 114(12):1629–1641
Yao Q, Wu Q, Xu X et al (2020) Resveratrol ameliorates systemic sclerosis via suppression of fibrosis and inflammation through activation of SIRT1/mTOR signaling. Drug Des Devel Ther 14:5337–5348
Zeng Z, Cheng S, Chen H et al (2017) Activation and overexpression of Sirt1 attenuates lung fibrosis via P300. Biochem Biophys Res Commun 486(4):1021–1026
Ramirez T, Li YM, Yin S et al (2017) Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol 66(3):601–609
Ramadori G, Lee CE, Bookout AL et al (2008) Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 28(40):9989–9996
Hernández-Jiménez M, Hurtado O, Cuartero MI et al (2013) Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 44(8):2333–2337
Li D, Liu N, Zhao HH et al (2017) Interactions between Sirt1 and MAPKs regulate astrocyte activation induced by brain injury in vitro and in vivo. J Neuroinflammation 14(1):67
Zhang Q, Zhang P, Qi GJ et al (2018) Cdk5 suppression blocks SIRT1 degradation via the ubiquitin-proteasome pathway in Parkinson’s disease models. Biochim Biophys Acta Gen Subj 1862(6):1443–1451
Yang R, Shen YJ, Chen M et al (2022) Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J Asian Nat Prod Res 24(3):278–289
Yu P, Wang L, Tang F et al (2017) Resveratrol pretreatment decreases ischemic injury and improves neurological function via sonic hedgehog signaling after stroke in rats. Mol Neurobiol 54(1):212–226
Chen J, Li Y, Wang L et al (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32(4):1005–1011
Zhang K, Zhang Q, Deng J et al (2019) ALK5 signaling pathway mediates neurogenesis and functional recovery after cerebral ischemia/reperfusion in rats via Gadd45b. Cell Death Dis 10(5):360
Gomes FC, Sousa Vde O, Romão L (2005) Emerging roles for TGF-beta1 in nervous system development. Int J Dev Neurosci 23(5):413–424
Andersen JL, Thompson JW, Lindblom KR et al (2011) A biotin switch-based proteomics approach identifies 14-3-3ζ as a target of Sirt1 in the metabolic regulation of caspase-2. Mol Cell 43(5):834–842
Mortenson JB, Heppler LN, Banks CJ et al (2015) Histone deacetylase 6 (HDAC6) promotes the pro-survival activity of 14-3-3ζ via deacetylation of lysines within the 14-3-3ζ binding pocket. J Biol Chem 290(20):12487–12496
Chu H, Jiang S, Liu Q et al (2018) Sirtuin1 Protects against Systemic Sclerosis-related Pulmonary Fibrosis by Decreasing Proinflammatory and Profibrotic Processes. Am J Respir Cell Mol Biol 58(1):28–39
Zhang Y, Huang W, Zheng Z et al (2021) Cigarette smoke-inactivated SIRT1 promotes autophagy-dependent senescence of alveolar epithelial type 2 cells to induce pulmonary fibrosis. Free Radic Biol Med 166:116–127
Li M, Hong W, Hao C et al (2018) SIRT1 antagonizes liver fibrosis by blocking hepatic stellate cell activation in mice. FASEB J 32(1):500–511
Bai XZ, Liu JQ, Yang LL et al (2016) Identification of sirtuin 1 as a promising therapeutic target for hypertrophic scars. Br J Pharmacol 173(10):1589–1601
Wei J, Ghosh AK, Chu H et al (2015) The Histone deacetylase sirtuin 1 is reduced in systemic sclerosis and abrogates fibrotic responses by targeting transforming growth factor β signaling. Arthritis Rheumatol 67(5):1323–1334
Zhu X, Chu H, Jiang S et al (2017) Sirt1 ameliorates systemic sclerosis by targeting the mTOR pathway. J Dermatol Sci 87(2):149–158
Sun HJ, Xiong SP, Cao X et al (2021) Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol 38:101813
Du L, Qian X, Li Y et al (2021) Sirt1 inhibits renal tubular cell epithelial-mesenchymal transition through YY1 deacetylation in diabetic nephropathy. Acta Pharmacol Sin 42(2):242–251
Kida Y, Zullo JA, Goligorsky MS (2016) Endothelial sirtuin 1 inactivation enhances capillary rarefaction and fibrosis following kidney injury through Notch activation. Biochem Biophys Res Commun 478(3):1074–1079
Li P, Liu Y, Qin X et al (2021) SIRT1 attenuates renal fibrosis by repressing HIF-2α. Cell Death Discov 7(1):59
Huang K, Huang J, Xie X et al (2013) Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med 65:528–540
Ren Y, Du C, Shi Y, Wei J, Wu H, Cui H (2017) The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int J Mol Med 39(5):1317–1324
Han L, Tang Y, Li S et al (2020) Protective mechanism of SIRT1 on Hcy-induced atrial fibrosis mediated by TRPC3. J Cell Mol Med 24(1):488–510
Chen Y, He T, Zhang Z, Zhang J (2021) Activation of SIRT1 by resveratrol alleviates pressure overload-induced cardiac hypertrophy via suppression of TGF-β1 signaling. Pharmacology 106(11–12):667–681
Wu WY, Cui YK, Hong YX et al (2020) Doxorubicin cardiomyopathy is ameliorated by acacetin via Sirt1-mediated activation of AMPK/Nrf2 signal molecules. J Cell Mol Med 24(20):12141–12153
Li Y, Xi Y, Tao G et al (2020) Sirtuin 1 activation alleviates primary biliary cholangitis via the blocking of the NF-κB signaling pathway. Int Immunopharmacol 83:106386
Yang L, Ao Q, Zhong Q, Li W, Li W (2020) SIRT1/IGFBPrP1/TGF β1 axis involved in cucurbitacin B ameliorating concanavalin A-induced mice liver fibrosis. Basic Clin Pharmacol Toxicol 127(5):371–379
Ma JQ, Sun YZ, Ming QL, Tian ZK, Yang HX, Liu CM (2019) Ampelopsin attenuates carbon tetrachloride-induced mouse liver fibrosis and hepatic stellate cell activation associated with the SIRT1/TGF-β1/Smad3 and autophagy pathway. Int Immunopharmacol 77:105984
Aitken A (2006) 14-3-3 proteins: a historic overview. Semin Cancer Biol 16(3):162–172
McGowan J, Peter C, Kim J et al (2020) 14-3-3ζ-TRAF5 axis governs interleukin-17A signaling. Proc Natl Acad Sci U S A 117(40):25008–25017
Shen C, Liu M, Xu R et al (2020) The 14-3-3ζ-c-Src-integrin-β3 complex is vital for platelet activation. Blood 136(8):974–988
Wang TT, Wu LL, Wu J et al (2023) 14-3-3ζ inhibits maladaptive repair in renal tubules by regulating YAP and reduces renal interstitial fibrosis. Acta Pharmacol Sin 44(2):381–392
Zhang X, Li LX, Yu C, Nath KA, Zhuang S, Li X (2022) Targeting lysine-specific demethylase 1A inhibits renal epithelial-mesenchymal transition and attenuates renal fibrosis. FASEB J 36(1):e22122
Wei L, Hu N, Ye M et al (2022) Overexpression of 14-3-3ζ primes disease recurrence, metastasis and resistance to chemotherapy by inducing epithelial-mesenchymal transition in NSCLC. Aging (Albany NY) 14(14):5838–5854
Zhao Y, Qiao W, Wang X et al (2016) 14-3-3ζ/TGFβR1 promotes tumor metastasis in lung squamous cell carcinoma. Oncotarget 7(50):82972–82984
Acknowledgements
We acknowledge and appreciate our colleagues for their valuable suggestions and technical assistance for this study.
Funding
This study was funded by the National Nature Science Foundation of China (Grant no. 81971229 and 82171456).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures were approved by the Ethics Committee of Chongqing Medical University.
Consent to Participate
Not applicable (no human research).
Consent for Publication
Not applicable (no human research).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Sirt1 expression is downregulated in the ischemic area of rats with MCAO/R injury and in the activated fibroblasts.
2. Sirt1 overexpression reduces fibrotic scar formation and improves neurological function in vivo.
3. Sirt1 overexpression inhibits the fibrotic reaction in fibroblasts in vitro through the deacetylation of 14–3-3ζ.
4. Sirt1 may be a novel therapeutic target of ischemic stroke by regulating fibrotic scarring.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Huang, J., Liu, J. et al. Sirt1 Overexpression Inhibits Fibrous Scar Formation and Improves Functional Recovery After Cerebral Ischemic Injury Through the Deacetylation of 14–3-3ζ. Mol Neurobiol 60, 4795–4810 (2023). https://doi.org/10.1007/s12035-023-03378-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03378-9