Abstract
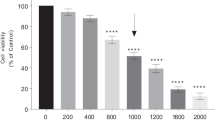
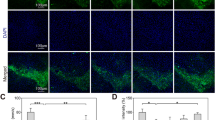
Parkinson’s disease (PD) is a progressive neurodegenerative condition. The pathogenesis of PD is still unknown, and drugs available for PD treatment either have side effects or have suboptimal efficacy. Flavonoids are potent antioxidants having little toxicity with extended use, suggesting they might hold promising therapeutic potential against PD. Vanillin (Van) is a phenolic compound that has exhibited neuroprotective properties in various neurological disorders, including PD. However, the neuroprotective role of Van in PD and its underlying mechanisms are scarce and therefore need more exploration. Here, we evaluated the neuroprotective potential of Van and its associated mechanisms against MPP+/MPTP-induced neuronal loss in differentiated human neuroblastoma (SH-SY5Y) cells and the mouse model of PD. In the present study, Van treatment significantly enhanced the cell viability and alleviated oxidative stress, mitochondrial membrane potential, and apoptosis in MPP+-intoxicated SH-SY5Y cells. Moreover, Van significantly ameliorated the MPP+-induced dysregulations in protein expression of tyrosine hydroxylase (TH) and mRNA expressions of GSK-3β, PARP1, p53, Bcl-2, Bax, and Caspase-3 genes in SH-SY5Y cells. Similar to our in vitro results, Van significantly alleviated MPTP-induced neurobehavioral dysregulations, oxidative stress, aberrant TH protein expressions, and immunoreactivity in SNpc of mice brains. Treatment of Van also prevented MPTP-mediated loss of TH-positive intrinsic dopaminergic neurons to SNpc and TH-fibers projecting to the striatum of mice. Thus, Van exhibited promising neuroprotective properties in the current study against MPP+/MPTP-intoxicated SH-SY5Y cells and mice, indicating its potential therapeutic properties against PD pathology.
Graphical abstract














Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included.
Abbreviations
- AO:
-
Acridine orange
- AD:
-
Alzheimer’s disease
- ANOVA:
-
One-way analysis of variance
- BSA:
-
Bovine serum albumin
- BHT:
-
Butylated hydroxytoluene
- CAT:
-
Catalase
- DCFH-DA:
-
2-7-Diacetyl dichlorofluorescein
- DCFH:
-
2′, 7′-Dichloro-dihydrofluorescin
- DCF:
-
2′,7′-Dichlorofluorescein
- DAB:
-
Diaminobenzidine
- DAT:
-
Dopamine transporters
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- Ellman’s reagent:
-
5,5′-Dithiobis-(2-nitrobenzoic acid)
- FBS:
-
Fetal bovine serum
- GSH:
-
Glutathione
- H&E:
-
Hematoxylin-Eosin
- SH-SY5Y:
-
Human neuroblastoma cells
- H2O2 :
-
Hydrogen peroxide
- LPO:
-
Lipid peroxidation
- Δψm:
-
Mitochondrial membrane potential
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+ :
-
1-Methyl-4-phenylpyridinium
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NGS:
-
Normal goat serum
- NO:
-
Nitric oxide
- 6-OHDA:
-
6-Hydroxydopamine
- OPA:
-
Orthophosphoric acid
- PFA:
-
Paraformaldehyde
- PD:
-
Parkinson’s disease
- PBS:
-
Phosphate buffer saline
- PI:
-
Propidium iodide
- PARP:
-
Poly (ADP-ribose) polymerase
- PMS:
-
Post mitochondrial supernatant
- Pyrogallol:
-
1,2,3-Trihydroxy benzene
- RA:
-
Retinoic acid
- ROS:
-
Reactive oxygen species
- SNpc:
-
Substantia nigra paras compacta
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TBARS:
-
Thiobarbituric acid reactive substance
- TH:
-
Tyrosine hydroxylase
- Van:
-
Vanillin
References
Dorsey ER, Sherer T, Okun MS, Bloem BR (2018) The emerging evidence of the Parkinson pandemic. J Parkinson’s Dis 8:S3–S8
Rizek P, Kumar N, Jog MS (2016) An update on the diagnosis and treatment of Parkinson disease. CMAJ 188:1157–1165
Del Rey NL-G, Quiroga-Varela A, Garbayo E, et al. Advances in Parkinson’s disease: 200 years later. Frontiers in Neuroanatomy 2018;12:113.
Grünewald A, Kumar KR, Sue CM (2019) New insights into the complex role of mitochondria in Parkinson’s disease. Prog Neurobiol 177:73–93
Zhu Y, Zhang J, Zeng Y (2012) Overview of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol Disord: Drug Targets 11:350–358
Shehadeh J, Double KL, Murphy KE, et al. Expression of tyrosine hydroxylase isoforms and phosphorylation at serine 40 in the human nigrostriatal system in Parkinson’s disease. 2019;130:104524.
Nagatsu T, Nakashima A, Ichinose H, Kobayashi KJJont. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. 2019;126:397-409
Chia SJ, Tan E-K, Chao Y-X (2020) Historical perspective: models of Parkinson’s disease. Int J Mol Sci 21:2464
Meredith GE, Rademacher DJ (2011) MPTP mouse models of Parkinson’s disease: an update. J Parkinsons Dis 1:19–33
Mustapha M, Mat Taib CN (2021) MPTP-induced mouse model of Parkinson’s disease: a promising direction of therapeutic strategies. Bosn J Basic Med Sci 21:422–433
Sun M-F, Zhu Y-L, Zhou Z-L, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. 2018;70:48–60.
McCarty MF, Lerner AJIJoMS. Nutraceuticals targeting generation and oxidant activity of peroxynitrite may aid prevention and control of Parkinson’s disease. 2020;21:3624.
Maiti P, Manna J, Dunbar GL (2017) Current understanding of the molecular mechanisms in Parkinson’s disease: targets for potential treatments. Transl Neurodegener 6:28
de Bie RMA, Clarke CE, Espay AJ, Fox SH, Lang AE (2020) Initiation of pharmacological therapy in Parkinson’s disease: when, why, and how. The Lancet Neurology 19:452–461
Fox SH, Katzenschlager R, Lim SY et al (2018) International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33:1248–1266
Forni C, Facchiano F, Bartoli M et al (2019) Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed Res Int 2019:8748253–8748253
Abuthawabeh R, Abuirmeileh AN, Alzoubi KH (2020) The beneficial effect of vanillin on 6-hydroxydopamine rat model of Parkinson’s disease. Restor Neurol Neurosci 38:369–373
Arya SS, Rookes JE, Cahill DM, Lenka SK (2021) Vanillin: a review on the therapeutic prospects of a popular flavouring molecule. Advances in Traditional Medicine 21:1–17
Dhanalakshmi C, Janakiraman U, Manivasagam T et al (2016) Vanillin attenuated behavioural impairments, neurochemical deficts, oxidative stress and apoptosis against rotenone induced rat model of Parkinson’s disease. Neurochem Res 41:1899–1910
Kumar SS, Priyadarsini KI, Sainis KB (2004) Inhibition of peroxynitrite-mediated reactions by Vanillin. J Agric Food Chem 52:139–145
Dhanalakshmi C, Manivasagam T, Nataraj J, Justin Thenmozhi A, Essa MM (2015) Neurosupportive role of Vanillin, a natural phenolic compound, on rotenone induced neurotoxicity in SH-SY5Y neuroblastoma cells. Evidence-based complementary and alternative Med eCAM 2015:626028
Korecka JA, van Kesteren RE, Blaas E, Spitzer SO, Kamstra JH, Smit AB, Swaab DF, Verhaagen J, Bossers K (2013) Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS ONE 8(5). https://doi.org/10.1371/journal.pone.0063862
Kim C, Park S (2018) IGF-1 protects SH-SY5Y cells against MPP(+)-induced apoptosis via PI3K/PDK-1/Akt pathway. Endocr Connect 7:443–455
Srivastav S, Anand BG, Fatima M et al (2020) Piperine-coated gold nanoparticles alleviate paraquat-induced neurotoxicity in Drosophila melanogaster. ACS Chem Neurosci 11:3772–3785
Fatima M, Ahmad MH, Srivastav S, Rizvi MA, Mondal AC (2020) A selective D2 dopamine receptor agonist alleviates depression through up-regulation of tyrosine hydroxylase and increased neurogenesis in hippocampus of the prenatally stressed rats. Neurochem Int 136:104730
Jackson-Lewis V, Przedborski S (2007) Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc 2:141–151
Matsuura K, Kabuto H, Makino H, Ogawa N (1997) Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods 73:45–48
Chonpathompikunlert P, Boonruamkaew P, Sukketsiri W, Hutamekalin P, Sroyraya M (2018) The antioxidant and neurochemical activity of Apium graveolens L and its ameliorative effect on MPTP-induced Parkinson-like symptoms in mice. BMC Complementary and Alternative Med 18:103
Paxinos G, Franklin KBJ (2008) The mouse brain in stereotaxic coordinates. Academic Press, Third
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Hilal Ahmad M, Fatima M, Hossain MM (2018) Mondal ACJJoB, Toxicology M Determination of potential oxidative damage, hepatotoxicity, and cytogenotoxicity in male Wistar rats. Role of Indomethacin 32:e22226
Ahmad MH, Fatima M, Ali M, Rizvi MA, Mondal AC (2021) Naringenin alleviates paraquat-induced dopaminergic neuronal loss in SH-SY5Y cells and a rat model of Parkinson’s disease. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2021.108831
Kumar S, Srivastav S, Fatima M, Giri RS, Mandal B (2019) Mondal ACJJoAsD A synthetic pro-drug peptide reverses amyloid-β-induced toxicity in the rat model of. Alzheimer’s Dis 69:499–512
Gundersen HJ, Bendtsen TF, Korbo L, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica 1988;96:379–394.
Mironov A (2017) Stereological morphometric grids for ImageJ. Ultrastruct Pathol 41:126
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Langston JW (2017) The MPTP Story. J Parkinson’s Dis 7:S11–S19
Aryal S, Skinner, T. Bridges, B. Weber, J. T. The pathology of Parkinson’s disease and potential benefit of dietary polyphenols. Molecules (Basel, Switzerland) 2020;25.
Yan X, Liu DF, Zhang XY, et al. Vanillin protects dopaminergic neurons against inflammation-mediated cell death by inhibiting ERK1/2, P38 and the NF-κB signaling pathway. Int J Mol Sci 2017;18.
Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Springer, Neuronal Cell Culture, pp 9–21
More SV, Choi D-KJIjoms. Atractylenolide-i protects human sh-sy5y cells from 1-methyl-4-phenylpyridinium-induced apoptotic cell death. 2017;18:1012.
Bezerra-Filho CSM, Barboza JN, Souza MTS, Sabry P, Ismail NSM, de Sousa DP (2019) Therapeutic potential of Vanillin and its main metabolites to regulate the inflammatory response and oxidative stress. Mini Rev Med Chem 19:1681–1693
Chanthammachat P, Dharmasaroja PJEj. Metformin restores the mitochondrial membrane potentials in association with a reduction in TIMM23 and NDUFS3 in MPP+-induced neurotoxicity in SH-SY5Y cells. 2019;18:812.
Yıldızhan K, Nazıroğlu M (2022) Protective role of selenium on MPP(+) and homocysteine-induced TRPM2 channel activation in SH-SY5Y cells. J Recept Signal Transduct Res 42:399–408
Naz H, Tarique M, Khan P et al (2018) Evidence of vanillin binding to CAMKIV explains the anti-cancer mechanism in human hepatic carcinoma and neuroblastoma cells. Mol Cell Biochem 438:35–45
Sefi M, Elwej A, Chaâbane M et al (2019) Beneficial role of vanillin, a polyphenolic flavoring agent, on maneb-induced oxidative stress. DNA damage, and liver histological changes in Swiss albino mice 38:619–631
Salau VFEO, Ibeji CU, Olasehinde TA, Koorbanally NA, Islam MS (2020) Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe(2+)-induced brain tissues damage. Metab Brain Dis 35:727–738
Oladimeji OH, Njinga S, Abdullahi ST. Evaluation of antioxidant activity of obtained derivatives of vanillin. Journal of Pharmaceutical Research Science & Technology [ISSN: 2583–3332] 2022;6:17–25.
Huang D, Xu J, Wang J, et al. dynamic changes in the nigrostriatal pathway in the MPTP mouse model of Parkinson’s disease. Parkinson’s Disease 2017;2017:9349487.
Torres ERS, Akinyeke T, Stagaman K et al (2018) Effects of sub-chronic MPTP exposure on behavioral and cognitive performance and the microbiome of wild-type and mGlu8 knockout female and male mice. Front Behav Neurosci 12:140
Abdulrahman AA, Faisal K, Meshref AAA, Arshaduddin M (2017) Low-dose acute vanillin is beneficial against harmaline-induced tremors in rats. Neurol Res 39:264–270
Rani L, Mondal AC (2020) Emerging concepts of mitochondrial dysfunction in Parkinson’s disease progression: pathogenic and therapeutic implications. Mitochondrion 50:25–34
Jiang P, Scarpa JR, Gao VD, Vitaterna MH, Kasarskis A, Turek FW (2019) Parkinson’s disease is associated with dysregulations of a dopamine-modulated gene network relevant to sleep and affective neurobehaviors in the striatum. Sci Rep 9:4808
Duda P, Wiśniewski J, Wójtowicz T et al (2018) Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin Ther Targets 22:833–848
Yang K, Chen Z, Gao J et al (2017) The key roles of GSK-3β in regulating mitochondrial activity. Cell Physiol Biochem 44:1445–1459
Li J, Ma S, Chen J et al (2020) GSK-3β contributes to parkinsonian dopaminergic neuron death: evidence from conditional knockout mice and tideglusib. Front Mol Neurosci 13:81
Zhang J, Liu W, Wang Y, Zhao S, Chang NJDm. miR-135b plays a neuroprotective role by targeting GSK3β in MPP+-intoxicated SH-SY5Y Cells. 2017;2017.
Ma W, Li X, Song P, et al. A vanillin derivative suppresses the growth of HT29 cells through the Wnt/β-catenin signaling pathway. 2019;849:43–49.
Pascal JM (2018) The comings and goings of PARP-1 in response to DNA damage. DNA Repair 71:177–182
Martire S, Mosca L, d’Erme MJMoa, development. PARP-1 involvement in neurodegeneration: a focus on Alzheimer’s and Parkinson’s diseases. 2015;146:53–64.
Mao K, Chen J, Yu H et al (2020) Poly (ADP-ribose) polymerase 1 inhibition prevents neurodegeneration and promotes α-synuclein degradation via transcription factor EB-dependent autophagy in mutant α-synucleinA53T model of Parkinson’s disease. Aging Cell 19:e13163
Espinoza-Derout J, Shao XM, Bankole E et al (2019) Hepatic DNA Damage induced by electronic cigarette exposure is associated with the modulation of NAD+/PARP1/SIRT1 Axis. Front Endocrinol 10:320
Enogieru AB, Haylett W, Hiss DC, Ekpo OEJMBD. Regulation of AKT/AMPK signaling, autophagy and mitigation of apoptosis in Rutin-pretreated SH-SY5Y cells exposed to MPP+. 2021;36:315–326.
Sirangelo IS, L. Ragone, A. Naviglio, S. Iannuzzi, C. Vanillin prevents doxorubicin-induced apoptosis and oxidative stress in rat H9c2 cardiomyocytes. 2020;12.
Lee Y-S, Kalimuthu K, Park YS, et al. BAX-dependent mitochondrial pathway mediates the crosstalk between ferroptosis and apoptosis. 2020;25:625-631.
Blandino G, Valenti F, Sacconi A, Di Agostino S. Wild type-and mutant p53 proteins in mitochondrial dysfunction: emerging insights in cancer disease. 2020 2020: Elsevier: 105–117.
Nkpaa KW, Awogbindin IO, Amadi BA, et al. Ethanol exacerbates manganese-induced neurobehavioral deficits, striatal oxidative stress, and apoptosis via regulation of p53, caspase-3, and bax/bcl-2 ratio-dependent pathway. 2019;191:135–148.
Liu H, Wang J, Zhang Q, Geng L, Yang Y, Wu N (2020) Protective effect of fucoidan against MPP+-induced SH-SY5Y cells apoptosis by affecting the PI3K/Akt pathway. Mar Drugs 18:333
Kawahata I, Fukunaga K. Degradation of tyrosine hydroxylase by the ubiquitin-proteasome system in the pathogenesis of Parkinson’s disease and dopa-responsive dystonia. Int J Mol Sci 2020;21.
Cobb CA, Cole MP (2015) Oxidative and nitrative stress in neurodegeneration. Neurobiol Dis 84:4–21
Chidambaram SB, Bhat A, Ray B, et al. Cocoa beans improve mitochondrial biogenesis via PPARγ/PGC1α dependent signalling pathway in MPP+ intoxicated human neuroblastoma cells (SH-SY5Y). 2020;23:471–480.
Roostalu U, Salinas CB, Thorbek DD, et al. Quantitative whole-brain 3D imaging of tyrosine hydroxylase-labeled neuron architecture in the mouse MPTP model of Parkinson’s disease. Disease models & mechanisms 2019;12:dmm042200.
Alam G, Edler M, Burchfield S, Richardson JR (2017) Single low doses of MPTP decrease tyrosine hydroxylase expression in the absence of overt neuron loss. Neurotoxicology 60:99–106
Peng S, Wang C, Ma J et al (2018) Achyranthes bidentata polypeptide protects dopaminergic neurons from apoptosis in Parkinson’s disease models both in vitro and in vivo. Br J Pharmacol 175:631–643
Acknowledgements
The authors would like to acknowledge the Central instrumentation facility, School of Life Sciences, Jawaharlal Nehru University, New Delhi, India. We are grateful to Prof. Ashis K. Nandi, School of Life Sciences, JNU, for his assistance in measuring the striatal MPP+ levels using HPLC.
Funding
This study was partially supported by a grant from DBT (BT/PR32907/MED/122/227/2019), DBT-SAHAJ/BUILDER (BT/INF/22/SP45382/2022) National Facility/Laboratory and DST-FIST-II [(SR/FST/LSII-046/2016(C)] to the School of Life Sciences, JNU. LR and MHA received financial grants from CSIR No. [09/263(1163)/2018-EMR-I] and ICMR (45/7/ 2019/MP/BMS), respectively.
Author information
Authors and Affiliations
Contributions
LR and MHA together designed and performed the experiments, helped analyze the results, and wrote and reviewed the manuscript. BG revised the manuscript. ACM conceptualized, supervised the study, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All the experiments were carried out on C57BL/6 mice following standard guidelines and regulations of the Institutional Animal Ethics Committee (IAEC), Jawaharlal Nehru University (JNU), New Delhi (10/GO/ReBi/99/CPCSEA/March 10, 1999). All the standard methods and protocols were followed for animal handling and experiments. All efforts were made to reduce animal suffering.
Consent to Participate
Not applicable.
Consent for Publication
All authors of the manuscript have agreed to publish this article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rani, L., Ghosh, B., Ahmad, M.H. et al. Evaluation of Potential Neuroprotective Effects of Vanillin Against MPP+/MPTP-Induced Dysregulation of Dopaminergic Regulatory Mechanisms in SH-SY5Y Cells and a Mouse Model of Parkinson’s Disease. Mol Neurobiol 60, 4693–4715 (2023). https://doi.org/10.1007/s12035-023-03358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03358-z