Abstract
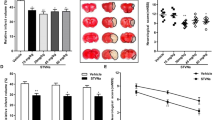
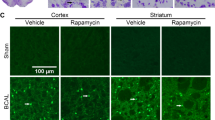
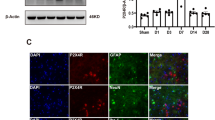
Pontine infarction is the major subtype of brainstem stroke causing severe neurological deficits. The pathophysiology and treatment of pontine infarction was rarely studied. A rat model of acute pontine infarction was established via injection of endothelin-1 in the pons. Single-cell RNA sequencing was applied to detect the cellular response in pontine infarction. Based on this finding, a potential treatment for pontine infarction targeting microglia was verified. Occlusion of penetrating artery caused by endothelin-1 led to pontine infarction. Single-cell RNA sequencing revealed a subtype of activated microglia, SPP1+ microglia, which were different from M1-like or M2-like depolarization. SPP1+ microglia interacted with oligodendrocytes and contributed to the demyelination of nerve tracts. Cyclin B1 regulated the proliferation of SPP1+ microglia. Cucurbitacin E, a cyclin B1 inhibitor, reduced the proliferation of SPP1+ microglia around the injured myelin sheath and alleviated the demyelination. Moreover, cucurbitacin E treatment decreased the ischemic infarction volume and neurological deficits after pontine infarction. SPP1+ microglia contributed to axonal demyelination in the pontine infarction, and inhibition of SPP1+ microglia provided neuroprotection for pontine infarction.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available in the GEO repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE199066 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE199067.
Abbreviations
- MCAO:
-
middle cerebral artery occlusion
- CST:
-
corticospinal tracts
- ET-1:
-
endothelin
- scRNA-seq:
-
single-cell RNA sequencing
- TR:
-
repetition time
- DWI:
-
diffusion-weighted imaging
- PWI:
-
perfusion-weighted imaging
- fMOST:
-
fluorescence micro-optical sectioning tomography
- CCA:
-
canonical correlation analysis
- NC:
-
normal control
- OPCs:
-
oligodendrocytes
- CuE:
-
cucurbitacin E
References
Li H, Kang Z, Qiu W et al (2012) Hemoglobin A1C is independently associated with severity and prognosis of brainstem infarctions. J Neurol Sci 317:87–91. https://doi.org/10.1016/j.jns.2012.02.024
Huang R, Zhang X, Chen W et al (2016) Stroke subtypes and topographic locations associated with neurological deterioration in acute isolated pontine infarction. J Stroke Cerebrovasc Dis 25:206–213. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.09.019
Xu X, Wen Z, Zhao N et al (2017) MicroRNA-1906, a novel regulator of toll-like receptor 4, ameliorates ischemic injury after experimental stroke in mice. J Neurosci 37:10498–10515. https://doi.org/10.1523/JNEUROSCI.1139-17.2017
Liu X, Dai Q, Ye R et al (2020) Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 19:115–122. https://doi.org/10.1016/S1474-4422(19)30395-3
Wojak JC, DeCrescito V, Young W (1991) Basilar artery occlusion in rats. Stroke 22:247–252. https://doi.org/10.1161/01.str.22.2.247
Liang Z, Zeng J, Zhang C et al (2008) Longitudinal Investigations on the anterograde and retrograde degeneration in the pyramidal tract following pontine infarction with diffusion tensor imaging. Cerebrovasc Dis 25:209–216. https://doi.org/10.1159/000113858
Hu X, Leak RK, Shi Y et al (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11:56–64. https://doi.org/10.1038/nrneurol.2014.207
Masuda T, Sankowski R, Staszewski O et al (2020) Microglia heterogeneity in the single-cell era. Cell Rep 30:1271–1281. https://doi.org/10.1016/j.celrep.2020.01.010
Li Q, Cheng Z, Zhou L et al (2019) Developmental Heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101(207-223):e210. https://doi.org/10.1016/j.neuron.2018.12.006
Hammond TR, Dufort C, Dissing-Olesen L et al (2019) Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50(253-271):e256. https://doi.org/10.1016/j.immuni.2018.11.004
Luo M, Tang X, Zhu J et al (2020) Establishment of acute pontine infarction in rats by electrical stimulation. J Vis Exp. https://doi.org/10.3791/60783
Lekic T, Rolland W, Manaenko A et al (2013) Evaluation of the hematoma consequences, neurobehavioral profiles, and histopathology in a rat model of pontine hemorrhage. J Neurosurg 118:465–477. https://doi.org/10.3171/2012.10.JNS111836
Jiang Y, Zhu J, Wu L et al (2012) Tetracycline inhibits local inflammation induced by cerebral ischemia via modulating autophagy. PLoS One 7:e48672. https://doi.org/10.1371/journal.pone.0048672
Zhang X, Yin X, Zhang J et al (2019) High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer’s disease mice. Natl Sci Rev 6:1223–1238. https://doi.org/10.1093/nsr/nwz124
Chen S, Zhou Y, Chen Y et al (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Ashburner M, Ball CA, Blake JA et al (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium Nat Genet 25:25–29. https://doi.org/10.1038/75556
Thomas L, Pasquini LA (2018) Galectin-3-mediated glial crosstalk drives oligodendrocyte differentiation and (re)myelination. Front Cell Neurosci 12:297. https://doi.org/10.3389/fncel.2018.00297
Zhou L, Yao M, Peng B et al (2018) Atherosclerosis might be responsible for branch artery disease: evidence from white matter hyperintensity burden in acute isolated pontine infarction. Front Neurol 9:840. https://doi.org/10.3389/fneur.2018.00840
Wilson LK, Pearce LA, Arauz A et al (2016) Morphological classification of penetrating artery pontine infarcts and association with risk factors and prognosis: the SPS3 trial. Int J Stroke 11:412–419. https://doi.org/10.1177/1747493016637366
Bassetti C, Bogousslavsky J, Barth A et al (1996) Isolated infarcts of the pons. Neurology 46:165–175
Safaiyan S, Besson-Girard S, Kaya T et al (2021) White matter aging drives microglial diversity. Neuron 109(1100-1117):e1110. https://doi.org/10.1016/j.neuron.2021.01.027
Gouna G, Klose C, Bosch-Queralt M et al (2021) TREM2-dependent lipid droplet biogenesis in phagocytes is required for remyelination. J Exp Med 218. https://doi.org/10.1084/jem.20210227
Cao Z, Harvey SS, Chiang T et al (2021) Unique subtype of microglia in degenerative thalamus after cortical stroke. Stroke 52:687–698. https://doi.org/10.1161/STROKEAHA.120.032402
Li T, Zhao J, Xie W et al (2021) Specific depletion of resident microglia in the early stage of stroke reduces cerebral ischemic damage. J Neuroinflammation 18:81. https://doi.org/10.1186/s12974-021-02127-w
Deng W, Mandeville E, Terasaki Y et al (2020) Transcriptomic characterization of microglia activation in a rat model of ischemic stroke. J Cereb Blood Flow Metab 40:S34–S48. https://doi.org/10.1177/0271678X20932870
Li Y, Dammer EB, Zhang-Brotzge X et al (2017) Osteopontin is a blood biomarker for microglial activation and brain injury in experimental hypoxic-ischemic encephalopathy. eNeuro 4. https://doi.org/10.1523/ENEURO.0253-16.2016
Schroeter M, Zickler P, Denhardt DT et al (2006) Increased thalamic neurodegeneration following ischaemic cortical stroke in osteopontin-deficient mice. Brain 129:1426–1437. https://doi.org/10.1093/brain/awl094
Sala Frigerio C, Wolfs L, Fattorelli N et al (2019) The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to abeta plaques. Cell Rep 27(1293-1306):e1296. https://doi.org/10.1016/j.celrep.2019.03.099
Willemsen L, de Winther MP (2020) Macrophage subsets in atherosclerosis as defined by single-cell technologies. J Pathol 250:705–714. https://doi.org/10.1002/path.5392
Masuda T, Sankowski R, Staszewski O et al (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566:388–392. https://doi.org/10.1038/s41586-019-0924-x
Mangale V, Syage AR, Ekiz HA et al (2020) Microglia influence host defense, disease, and repair following murine coronavirus infection of the central nervous system. Glia 68:2345–2360. https://doi.org/10.1002/glia.23844
Deng L, Wu RA, Sonneville R et al (2019) Mitotic CDK promotes replisome disassembly, fork breakage, and complex DNA rearrangements. Mol Cell 73(915-929):e916. https://doi.org/10.1016/j.molcel.2018.12.021
Mohamed TMA, Ang YS, Radzinsky E et al (2018) Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 173(104-116):e112. https://doi.org/10.1016/j.cell.2018.02.014
Böttcher C, Schlickeiser S, Sneeboer MAM et al (2018) Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci 22:78–90. https://doi.org/10.1038/s41593-018-0290-2
Kaushik U, Aeri V, Mir SR (2015) Cucurbitacins – an insight into medicinal leads from nature. Pharmacogn Rev 9:12–18. https://doi.org/10.4103/0973-7847.156314
Song H, Sui H, Zhang Q et al (2020) Cucurbitacin E induces autophagy-involved apoptosis in intestinal epithelial cells. Front Physiol 11:1020. https://doi.org/10.3389/fphys.2020.01020
Si W, Lyu J, Liu Z et al (2019) Cucurbitacin E inhibits cellular proliferation and enhances the chemo-response in gastric cancer by suppressing AKt activation. J Cancer 10:5843–5851. https://doi.org/10.7150/jca.31303
Shang J, Liu W, Yin C et al (2019) Cucurbitacin E ameliorates lipopolysaccharide-evoked injury, inflammation and MUC5AC expression in bronchial epithelial cells by restraining the HMGB1-TLR4-NF-kappaB signaling. Mol Immunol 114:571–577. https://doi.org/10.1016/j.molimm.2019.09.008
Attard E, Martinoli MG (2015) Cucurbitacin E, an experimental lead triterpenoid with anticancer, immunomodulatory and novel effects against degenerative diseases. A mini-review. Curr Top Med Chem 15:1708–1713. https://doi.org/10.2174/1568026615666150427121331
Liu Z, Kumar M, Devi S et al (2021) The mechanisms of cucurbitacin E as a neuroprotective and memory-enhancing agent in a cerebral hypoperfusion rat model: attenuation of oxidative stress, inflammation, and excitotoxicity. Front Pharmacol 12:794933. https://doi.org/10.3389/fphar.2021.794933
Acknowledgements
We thank Dr. Jiahui Liang and Dr. Wang for the help in DTI reconstruction, Dr. Weidong Chen for the help in the analysis of scRNA-seq, and Dr. Cheng Yan for the help in fMOST analysis.
Funding
This study was supported by the National Science Foundation of China (81870933), the GuangDong Basic and Applied Basic Research Foundation (2021A1515012351), the Guangzhou Basic and Applied Basic Research Foundation (202102010127), and the Opening Lab Program of Guangzhou Medical University (0506308) to Y. Jiang; the National Science Foundation of China (82171282), the Scientific Program of Jiangsu Provincial Health Commission (M2020064), and the Suzhou Science and Technology Program (SYS2020101) to J. Zhu; and the Scientific Program of Guangzhou Municipal Health Commission (20191A011083) to Z. Qiu.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Ming Luo, Zhihua Qiu, Xiangyue Tang, Li Wu, and Shaojun Li. The first draft of the manuscript was written by Yongjun Jiang and Juehua Zhu, and all authors commented on the previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The Second Affiliated Hospital of Guangzhou Medical University.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The authors affirm that this manuscript did not contain individual person’s data.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Figure S1
Overview of the single cells from pontine infarction and normal pontine samples. (A) Summary of the sample origins. Red area was the infarct zone and the blue area was the PWI-DWI mismatch. The samples were taken from the peri-infarct zone and the corresponding area in the NC group. (B) tSNE of the cells profiled here with each cell color-coded for (left to right): the sample origin (red from NC and blue from PI), the corresponding rat, the associated cell type, and the number of transcripts (UMIs) detected in the cell (log scale as defined in the inset). (C) Expression of marker genes for the cell types defined above panel. (D) For the 15 cell subclusters: the fraction of cells originating from each rat (upper left), from PI and NC (upper right), the number of cells (down left) and the bot plots of the number of transcripts (down right). PI, pontine infarction; NC, normal control. (PNG 2692 kb)

Figure S2
RNA sequencing between pontine infarction and MCAO. (A) Six hours after MCAO or PI, the tissues from the peri-infarct zone (mismatch of red dot line and blue area) or the corresponding area in the control groups were extracted for RNA-seq. (B) The down and up-regulated genes in the MCAO and PI compared to the normal controls. (C) The 478 genes were significantly changed in the PI peri-infarct zone. Of them, there were 377 up-regulated genes. (D) Pathway analysis showed that cell cycle had the most up-regulated genes. (E) Volcano plot of genes in the cell cycle. (F) Results of real-time PCR of genes in the cell cycle. PI, pontine infarction. *P<0.05 considered as significant difference when compared to the control group. (PNG 911 kb)

Figure S3
CuE inhibited the SPP1+ microglia in vitro. (A) CuE decreased the SPP1+ microglia. The Iba-1, CD68 and CD206 positive microglia showed no significant difference between OGD and CuE groups. (B) summarizes the positive cells. NC, normal control; OGD, oxygen glucose deprivation. * compared with NC; # compared with OGD. The scale bar represents 25 μm. (PNG 1714 kb)

Figure S4
Luxol Fast Blue (LFB) staining. The blue box was ischemic core, the red box was peri-infarction zone and the black box was normal area. The rats without injection of ET-1 (0h) as the control. At 6h, 24h, 3d, 7d and 14d after stroke onset, the rats were sacrificed for brain sections (20μm) and LFB staining. MCAO, middle cerebral artery occlusion; PI, pontine infarction. Scale bar was 10μm. (PNG 3932 kb)

Supplementary file 1
(PNG 4321 kb)
3D structure of pontine vessels. (MP4 11793 kb)
Comparison of occluded and non-occluded pontine vessels. (MP4 14649 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, M., Qiu, Z., Tang, X. et al. Inhibiting Cyclin B1-treated Pontine Infarction by Suppressing Proliferation of SPP1+ Microglia. Mol Neurobiol 60, 1782–1796 (2023). https://doi.org/10.1007/s12035-022-03183-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03183-w