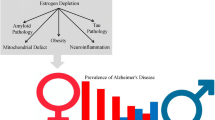
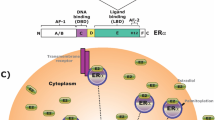
Abstract
Post-menopausal women are at a higher risk of developing Alzheimer’s disease (AD) than males. The higher rates of AD in women are associated with the sharp decline in the estrogen levels after menopause. Estrogen has been shown to downregulate inflammatory cytokines in the central nervous system (CNS), which has a neuroprotective role against neurodegenerative diseases including AD. Sustained neuroinflammation is associated with neurodegeneration and contributes to AD. Nuclear factor kappa-B (NF-κB) is a transcription factor involved with the modulation of inflammation and interacts with estrogen to influence the progression of AD. Application of 17β-estradiol (E2) has been shown to inhibit NF-κB, thereby reducing transcription of NF-κB target genes. Despite accumulating evidence showing that estrogens have beneficial effects in pre-clinical AD studies, there are mixed results with hormone replacement therapy in clinical trials. Furthering our understanding of how NF-κB interacts with estrogen and alters the progression of neurodegenerative disorders including AD, should be beneficial and result in the development of novel therapeutics.


Similar content being viewed by others
Data Availability
This is a review paper and no data was collected for this manuscript. However, the material supporting this paper is available in the article or is available from the corresponding author upon request.
References
Gatz M et al (2006) Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63(2):168–174
Djordjevic J, Sabbir MG, Albensi BC (2016) Traumatic brain injury as a risk factor for Alzheimer’s disease: is inflammatory signaling a key player?. Curr Alzheimer Res 13(7):730–738
Bendlin BB et al (2010) Midlife predictors of Alzheimer’s disease. Maturitas 65(2):131–137
James BD et al (2011) Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age. Am J Geriatr Psychiatry 19(11):961–969
Marwit SJ, Meuser TM (2002) Development and initial validation of an inventory to assess grief in caregivers of persons with Alzheimer’s disease. Gerontologist 42(6):751–765
Glabe CC (2005) Amyloid accumulation and pathogensis of Alzheimer’s disease: significance of monomeric, oligomeric and fibrillar Abeta. Subcell Biochem 38:167–177
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Muralidar S et al (2020) Role of tau protein in Alzheimer’s disease: the prime pathological player. Int J Biol Macromol
Adlimoghaddam A et al (2019) Regional hypometabolism in the 3xTg mouse model of Alzheimer’s disease. Neurobiol Dis 127:264–277
Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Sci 314(5800):777–781
Shoghi-Jadid K et al (2002) Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry 10(1):24–35
Ellis RJ et al (1996) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience Part XV. Neurol 46(6):1592–1596
Price JL et al (2001) Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol 58(9):1395–1402
Doody RS et al (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370(4):311–321
Abushouk AI et al (2017) Bapineuzumab for mild to moderate Alzheimer’s disease: a meta-analysis of randomized controlled trials. BMC Neurol 17(1):66
Sabbagh MN, Cummings J (2021) open peer commentary to “failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by biogen December 2019”. Alzheimers Dement 17(4):702–703
Fuentes N and Silveyra P (2019) Chapter three - estrogen receptor signaling mechanisms, in advances in protein chemistry and structural biology, R. Donev, Editor. Academic Press 135–170
Paterni I et al (2014) Estrogen receptors alpha (ERalpha) and beta (ERbeta): subtype-selective ligands and clinical potential. Steroids 90:13–29
Paterni I et al (2014) Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids 90:13–29
Zhao C, Dahlman-Wright K, Gustafsson JA (2008) Estrogen receptor beta: an overview and update. Nucl Recept Signal 6:e003
Hadjimarkou MM, Vasudevan N (2018) GPER1/GPR30 in the brain: crosstalk with classical estrogen receptors and implications for behavior. J Steroid Biochem Mol Biol 176:57–64
Revankar CM et al (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Sci 307(5715):1625–1630
Driscoll MD et al (1998) Sequence requirements for estrogen receptor binding to estrogen response elements. J Biol Chem 273(45):29321–29330
Gruber CJ et al (2004) Anatomy of the estrogen response element. Trends Endocrinol Metab 15(2):73–78
Gaub MP et al (1990) Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell 63(6):1267–1276
Webb P et al (1995) Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9(4):443–456
Sabbah M et al (1999) Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc Natl Acad Sci U S A 96(20):11217–11222
Tee MK et al (2004) Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell 15(3):1262–1272
Almey A, Milner TA, Brake WG (2015) Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 74:125–138
Papka RE, Mowa CN (2003) Estrogen receptors in the spinal cord, sensory ganglia, and pelvic autonomic ganglia. Int Rev Cytol 231:91–127
Compton J, van Amelsvoort T, Murphy D (2001) HRT and its effect on normal ageing of the brain and dementia. Br J Clin Pharmacol 52(6):647–653
Bean LA, Ianov L, Foster TC (2014) Estrogen receptors, the hippocampus, and memory. Neuroscientist 20(5):534–545
Pompili A, Iorio C, Gasbarri A (2020) Effects of sex steroid hormones on memory. Acta Neurobiol Exp (Wars) 80(2):117–128
Hara Y et al (2015) Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev 95(3):785–807
Handa RJ, Mani SK, Uht RM (2012) Estrogen receptors and the regulation of neural stress responses. Neuroendocrinol 96(2):111–118
Frick KM, Kim J, Koss WA (2018) Estradiol and hippocampal memory in female and male rodents. Curr Opin Behav Sci 23:65–74
Heldring N et al (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87(3):905–931
Kim CK, et al (2018) Structural and functional characteristics of oestrogen receptor beta splice variants: implications for the ageing brain. J Neuroendocrinol 30(2)
Ishunina TA, Swaab DF (2008) Estrogen receptor-alpha splice variants in the human brain. Gynecol Endocrinol 24(2):93–98
Ishunina TA, Fischer DF, Swaab DF (2007) Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer’s disease. Neurobiol Aging 28(11):1670–1681
Cui J, Shen Y, Li R (2013) Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med 19(3):197–209
Zhu D, Montagne A, Zhao Z (2021) Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell Mol Life Sci 78(11):4907–4920
Baum LW (2005) Sex, hormones, and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 60(6):736–743
Gandy S, Duff K (2000) Post-menopausal estrogen deprivation and Alzheimer’s disease. Exp Gerontol 35(4):503–511
Nilsen J et al (2006) Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci 7(1):74
Irwin RW et al (2008) Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinol 149(6):3167–3175
Goodman Y et al (1996) Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem 66(5):1836–1844
Vincent B, Smith JD (2000) Effect of estradiol on neuronal Swedish-mutated beta-amyloid precursor protein metabolism: reversal by astrocytic cells. Biochem Biophys Res Commun 271(1):82–85
Jaffe AB et al (1994) Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem 269(18):13065–13068
Xu H et al (1998) Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med 4(4):447–451
Green PS, Gridley KE, Simpkins JW (1996) Estradiol protects against beta-amyloid (25–35)-induced toxicity in SK-N-SH human neuroblastoma cells. Neurosci Lett 218(3):165–168
Li R et al (2000) Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem 75(4):1447–1454
Stephan A, Laroche S, Davis S (2001) Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci 21(15):5703–5714
Parent A et al (1999) Synaptic transmission and hippocampal long-term potentiation in transgenic mice expressing FAD-linked presenilin 1. Neurobiol Dis 6(1):56–62
Zaman SH et al (2000) Enhanced synaptic potentiation in transgenic mice expressing presenilin 1 familial Alzheimer’s disease mutation is normalized with a benzodiazepine. Neurobiol Dis 7(1):54–63
Wong M, Moss RL (1992) Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci 12(8):3217–3225
CordobaMontoya DA, Carrer HF (1997) Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res 778(2):430–8
Chavez C et al (2010) The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res 1321:51–59
Gross KS, Mermelstein PG (2020) Estrogen receptor signaling through metabotropic glutamate receptors. Vitam Horm 114:211–232
Del Rio JP et al (2018) Steroid hormones and their action in women’s brains: the importance of hormonal balance. Front Public Health 6:141
Jamshed N et al (2014) Alzheimer disease in post-menopausal women: intervene in the critical window period. J Midlife Health 5(1):38–40
Honjo H et al (1989) In vivo effects by estrone sulfate on the central nervous system-senile dementia (Alzheimer’s type). J Steroid Biochem 34(1–6):521–525
Ohkura T et al (1995) Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia 6(2):99–107
Cholerton B et al (2002) Estrogen and Alzheimer’s disease: the story so far. Drugs Aging 19(6):405–427
Zhou C et al (2020) The effect of hormone replacement therapy on cognitive function in female patients with Alzheimer’s disease: a meta-analysis. Am J Alzheimers Dis Other Demen 35:1533317520938585
Singh M, Su C (2013) Progesterone and neuroprotection. Horm Behav 63(2):284–290
Hong Y et al (2016) Progesterone exerts neuroprotective effects against Abeta-induced neuroinflammation by attenuating ER stress in astrocytes. Int Immunopharmacol 33:83–89
Hong Y et al (2019) The neuroprotection of progesterone against Abeta-induced NLRP3-Caspase-1 inflammasome activation via enhancing autophagy in astrocytes. Int Immunopharmacol 74:105669
Hong Y et al (2018) Progesterone suppresses Abeta42-induced neuroinflammation by enhancing autophagy in astrocytes. Int Immunopharmacol 54:336–343
Carroll JC et al (2007) Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27(48):13357–13365
Wang JM et al (2010) Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 107(14):6498–6503
Singh C et al (2012) Allopregnanolone restores hippocampal-dependent learning and memory and neural progenitor survival in aging 3xTgAD and nonTg mice. Neurobiol Aging 33(8):1493–1506
Kinney JW et al (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 4:575–590
Luchetti S, Huitinga I, Swaab D (2011) Neurosteroid and GABA-A receptor alterations in Alzheimer’s disease Parkinson’s disease and multiple sclerosis. Neurosci 191:6–21
Naylor JC et al (1801) 2010 Allopregnanolone levels are reduced in temporal cortex in patients with Alzheimer’s disease compared to cognitively intact control subjects. Biochimica et Biophysica Acta (BBA)-Mol Cell Biol Lipids 8:951–959
Yilmaz C et al (2019) Neurosteroids as regulators of neuroinflammation. Front Neuroendocrinol 55:100788
Ito A et al (2001) Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol 167(1):542–552
Bebo BF Jr et al (2001) Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol 166(3):2080–2089
Garidou L et al (2004) Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol 173(4):2435–2442
Benedek G et al (2017) Novel feedback loop between M2 macrophages/microglia and regulatory B cells in estrogen-protected EAE mice. J Neuroimmunol 305:59–67
Matejuk A et al (2001) 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res 65(6):529–542
Saijo K et al (2011) An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 145(4):584–595
Spence RD et al (2013) Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. J Neurosci 33(26):10924–10933
Spence RD et al (2011) Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A 108(21):8867–8872
Gadani SP et al (2015) Dealing with danger in the CNS: the response of the immune system to injury. Neuron 87(1):47–62
Yao X et al (2014) Estrogen-provided cardiac protection following burn trauma is mediated through a reduction in mitochondria-derived DAMPs. Am J Physiol-Heart Circ Physiol 306(6):H882–H894
Yun J et al (2018) Estrogen deficiency exacerbates Abeta-induced memory impairment through enhancement of neuroinflammation, amyloidogenesis and NF-kB activation in ovariectomized mice. Brain Behav Immun 73:282–293
Vegeto E et al (2006) The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinol 147(5):2263–2272
Slowik A et al (2018) Impact of steroid hormones E2 and P on the NLRP3/ASC/Casp1 axis in primary mouse astroglia and BV-2 cells after in vitro hypoxia. J Steroid Biochem Mol Biol 183:18–26
Reed JL et al (2004) Estrogen increases proteasome activity in murine microglial cells. Neurosci Lett 367(1):60–65
Farrer LA et al (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease A meta-analysis APOE and Alzheimer disease meta analysis consortium. JAMA 278(16):1349–56
Davignon J, Gregg RE, Sing CF (1988) Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 8(1):1–21
Safieh M, Korczyn AD, Michaelson DM (2019) ApoE4: an emerging therapeutic target for Alzheimer’s disease. BMC Med 17(1):64
Mahley RW, Rall SC Jr (2000) Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1:507–537
Villa A et al (2016) Estrogens, Neuroinflammation, and Neurodegeneration. Endocr Rev 37(4):372–402
Davies DA (2021) The role of APOE and NF-κB in Alzheimer’s disease. Immuno 1(4):391–399
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344
Newcombe EA et al (2018) Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation 15(1):276
Bertram L, Tanzi RE (2008) Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 9(10):768–778
Gale SC et al (2014) APOepsilon4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol 134(1):127–134
Olgiati P et al (2010) APOE epsilon-4 allele and cytokine production in Alzheimer’s disease. Int J Geriatr Psychiatry 25(4):338–344
Jacobs EG et al (2013) Accelerated cell aging in female APOE-epsilon4 carriers: implications for hormone therapy use. PLoS One 8(2):e54713
Kantarci K et al (2016) Early postmenopausal transdermal 17β-estradiol therapy and amyloid-β deposition. J Alzheimer’s Dis 53:547–556
Brown CM et al (2008) The APOE4 genotype alters the response of microglia and macrophages to 17beta-estradiol. Neurobiol Aging 29(12):1783–1794
Wang JM, Irwin RW, Brinton RD (2006) Activation of estrogen receptor α increases and estrogen receptor β decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci 103(45):16983–16988
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651
Eshraghi M et al (2021) Alzheimer’s disease pathogenesis: role of autophagy and mitophagy focusing in microglia. Int J Mol Sci 22(7)
Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18(18):2195–2224
Albensi BC (2019) What is nuclear factor kappa B (NF-kappaB) doing in and to the mitochondrion? Front Cell Dev Biol 7:154
Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2(10):725–734
Kaltschmidt B et al (2022) NF-κB in neurodegenerative diseases: recent evidence from human genetics. Front Mol Neurosci 15:954541
O’Neill LA, Kaltschmidt C (1997) NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci 20(6):252–258
Granic I et al (2009) Inflammation and NF-kappaB in Alzheimer’s disease and diabetes. J Alzheimers Dis 16(4):809–821
Goel D, Vohora D (2021) Liver X receptors and skeleton: current state-of-knowledge. Bone 144:115807
Kaltschmidt B et al (1997) Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci USA 94(6):2642–2647
Huang P et al (2020) Curcumin inhibits BACE1 expression through the interaction between ERbeta and NFkappaB signaling pathway in SH-SY5Y cells. Mol Cell Biochem 463(1–2):161–173
Zheng N et al (2015) Luteolin reduces BACE1 expression through NF-kappaB and through estrogen receptor mediated pathways in HEK293 and SH-SY5Y Cells. J Alzheimers Dis 45(2):659–671
Hwang CJ et al (2016) Acceleration of amyloidogenesis and memory impairment by estrogen deficiency through NF-kappaB dependent beta-secretase activation in presenilin 2 mutant mice. Brain Behav Immun 53:113–122
Dodel RC et al (1999) Sodium salicylate and 17beta-estradiol attenuate nuclear transcription factor NF-kappaB translocation in cultured rat astroglial cultures following exposure to amyloid A beta(1–40) and lipopolysaccharides. J Neurochem 73(4):1453–1460
Vegeto E, Benedusi V, Maggi A (2008) Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol 29(4):507–519
Ghisletti S et al (2005) 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol 25(8):2957–2968
Jung YJ et al (2003) Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. J Biol Chem 278(9):7445–7452
Rosette C, Karin M (1995) Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. J Cell Biol 128(6):1111–1119
Snezhkina AV et al (2019) ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev 2019:6175804
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426
Cullinan SB et al (2004) The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol 24(19):8477–8486
Kobayashi A et al (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24(16):7130–7139
Zhang DD et al (2004) Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol 24(24):10941–10953
Furukawa M, Xiong Y (2005) BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol 25(1):162–171
Wakabayashi N et al (2004) Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A 101(7):2040–2045
Dinkova-Kostova AT et al (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 99(18):11908–11913
Dinkova-Kostova AT, Holtzclaw WD, Wakabayashi N (2005) Keap1, the sensor for electrophiles and oxidants that regulates the phase 2 response, is a zinc metalloprotein. Biochem 44(18):6889–6899
Eggler AL et al (2005) Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci USA 102(29):10070–10075
Tong KI et al (2006) Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem 387(10–11):1311–1320
Kobayashi A et al (2006) Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol 26(1):221–229
Yamamoto M, Kensler TW, Motohashi H (2018) The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev 98(3):1169–1203
Davies DA, A Adlimoghaddam, BC Albensi (2021) Role of Nrf2 in synaptic plasticity and memory in Alzheimer’s disease. Cells 10(8)
Khan I et al (2021) 17-Beta estradiol rescued immature rat brain against glutamate-induced oxidative stress and neurodegeneration via regulating Nrf2/HO-1 and MAP-kinase signaling pathway. Antioxidants (Basel) 10(6)
Khan M et al (2019) 17-Beta-estradiol modulates SIRT1 and halts oxidative stress-mediated cognitive impairment in a male aging mouse model. Cells 8(8)
Wu J et al (2014) Estrogen increases Nrf2 activity through activation of the PI3K pathway in MCF-7 breast cancer cells. Exp Cell Res 328(2):351–360
Song CH et al (2019) 17-beta estradiol exerts anti-inflammatory effects through activation of Nrf2 in mouse embryonic fibroblasts. PLoS ONE 14(8):e0221650
Kong D et al (2019) Effects of resveratrol on the mechanisms of antioxidants and estrogen in Alzheimer’s disease. Biomed Res Int 2019:8983752
Jantaratnotai N et al (2013) Phytoestrogens mediated anti-inflammatory effect through suppression of IRF-1 and pSTAT1 expressions in lipopolysaccharide-activated microglia. Int Immunopharmacol 17(2):483–488
Kaplan MH (2013) STAT signaling in inflammation. JAKSTAT 2(1):e24198
Platanitis E, Decker T (2018) Regulatory networks involving STATs, IRFs, and NFkappaB in inflammation. Front Immunol 9:2542
Wong CK et al (2012) Estrogen controls embryonic stem cell proliferation via store-operated calcium entry and the nuclear factor of activated T-cells (NFAT). J Cell Physiol 227(6):2519–2530
Abdul HM et al (2010) NFATs and Alzheimer’s disease. Mol Cell Pharmacol 2(1):7–14
Maiese K (2016) Forkhead transcription factors: new considerations for Alzheimer’s disease and dementia. J Transl Sci 2(4):241–247
Tower J, Pomatto LCD, Davies KJA (2020) Sex differences in the response to oxidative and proteolytic stress. Redox Biol 31:101488
Webster KM et al (2015) Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J Neuroinflammation 12(1):238
Feng R et al (2022) Progesterone regulates inflammation and receptivity of cells via the NF-kappaB and LIF/STAT3 pathways. Theriogenol 186:50–59
Hardy DB et al (2006) Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol 20(11):2724–2733
Kalkhoven E et al (1996) Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem 271(11):6217–6224
Cui L et al (2022) Anti-inflammatory effects of progesterone through NF-kappaB and MAPK pathway in lipopolysaccharide- or Escherichia coli-stimulated bovine endometrial stromal cells. PLoS One 17(4):e0266144
Abdi F et al (2016) Hormone therapy for relieving postmenopausal vasomotor symptoms: a systematic review. Archives of Iranian Medicine 19(2): 0–0.
Sturdee D, Panay NA (2010) Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 13(6):509–522
Gambacciani M, Levancini M (2014) Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz Menopauzalny 13(4):213–220
Asthana S et al (1999) Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinol 24(6):657–677
Wharton W et al (2011) Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer’s disease: results of a randomized controlled trial. J Alzheimers Dis 26(3):495–505
Zandi PP et al (2002) Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288(17):2123–2129
Waring SC et al (1999) Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurol 52(5):965–970
Tang MX et al (1996) Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 348(9025):429–432
Verma E et al (2021) Potential of baicalein in the prevention and treatment of cancer: a scientometric analyses based review. J Funct Foods 86:104660
Vinogradova Y et al (2021) Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ 374:n2182
Song YJ et al (2020) The effect of estrogen replacement therapy on Alzheimer’s disease and Parkinson’s disease in postmenopausal women: a meta-analysis. Front Neurosci 14:157
Mulnard RA et al (2000) Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial Alzheimer’s disease cooperative study. JAMA 283(8):1007–15
Shumaker SA et al (2004) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291(24):2947–2958
Shumaker SA et al (2003) Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289(20):2651–2662
Savolainen-Peltonen H et al (2019) Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ 364:l665
Shao H et al (2012) Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurol 79(18):1846–1852
Kunzler J et al (2014) APOE modulates the effect of estrogen therapy on Abeta accumulation EFAD-Tg mice. Neurosci Lett 560:131–136
Maki PM (2013) Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 20(6):695–709
Zhang QG et al (2011) C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci U S A 108(35):E617–E624
Acknowledgements
B.C.A. previously held the Manitoba Dementia Research Chair (funded by the Alzheimer’s Soc. of Manitoba and Research Manitoba) and the Honourable Douglas and Patricia Everett, Royal Canadian Properties Limited Endowment Chair.
Funding
We gratefully acknowledge the support from the St. Boniface Hospital Research Foundation (Grant Nos. 1406–3216 and 1410–3216), the Canadian Institute of Health Research (CIHR; Grant No. PJT-162144) to B.C.A., the Alzheimer’s Society of Manitoba, the Honourable Douglas and Patricia Everett, Royal Canadian Properties Limited Endowment Fund (Grant No. 1403–3131) to B.C.A. B.C.A. previously held the Manitoba Dementia Research Chair (funded by the Alzheimer’s Soc. of Manitoba and Research Manitoba).
Author information
Authors and Affiliations
Contributions
Pranav Mishra and Don Davies created the outline and wrote much of the paper. Benedict C. Albensi supervised the writing, edited the manuscript, provided additional text and references when necessary.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Authors consent to publish identifiable details within the text in the journal of molecular neurobiology.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pranav Mishra and Don a. Davies are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, P., Davies, D.A. & Albensi, B.C. The Interaction Between NF-κB and Estrogen in Alzheimer’s Disease. Mol Neurobiol 60, 1515–1526 (2023). https://doi.org/10.1007/s12035-022-03152-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03152-3