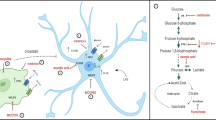
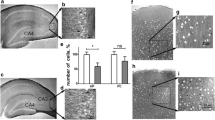
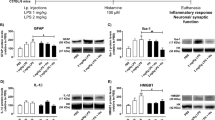
Abstract
Inflammation has been associated with numerous neurological disorders. Inflammatory environments trigger a series of cellular and physiological alterations in the brain. However, how inflammatory milieu affects neuronal physiology and how neuronal alterations progress in the inflammatory environments are not fully understood. In this study, we examined the effects of pro-inflammatory milieu on mitochondrial functions and neuronal activities in the hypothalamic POMC neurons. Treating mHypoA-POMC/GFP1 with the conditioned medium collected from LPS activated macrophage were employed to mimic the inflammatory milieu during hypothalamic inflammation. After a 24-h treatment, intracellular ROS/RNS levels were elevated, and the antioxidant enzymes were reduced. Mitochondrial respiration and mitochondrial functions, including basal respiratory rate, spared respiration capacity, and maximal respiration, were all significantly compromised by inflammatory milieu. Moreover, pro-inflammatory cytokines altered mitochondrial dynamics in a time-dependent manner, resulting in the elongation of mitochondria in POMC neurons after a 24-h treatment. Additionally, the increase of C-Fos and Pomc genes expression indicated that the neurons were activated upon the stimulation of inflammatory environment. This neuronal activation of were confirmed on the LPS-challenged mice. Collectively, a short-term to midterm exposure to inflammatory milieu stimulated metabolic switch and neuronal activation, whereas chronic exposure triggered the elevation of oxidative stress, the decrease of the mitochondrial respiration, and the alterations of mitochondrial dynamics.







Similar content being viewed by others
Data Availability
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lurie DI (2018) An integrative approach to neuroinflammation in psychiatric disorders and neuropathic pain. J Exp Neurosci 12:1–11
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N (2019) Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol 10:1008
DiSabato DJ, Quan N, Godbout JP (2016) Neuroinflammation: the devil is in the details. J Neurochem 139(Suppl 2):136–153
del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO (2013) A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immun 33:15–23
Janelidze S, Mattei D, Westrin A, Traskman-Bendz L, Brundin L (2011) Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun 25:335–339
Schnieder TP, Trencevska I, Rosoklija G, Stankov A, Mann JJ, Smiley J, Dwork AJ (2014) Microglia of prefrontal white matter in suicide. J Neuropathol Exp Neurol 73:880–890
Timper K, Bruning JC (2017) Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech 10:679–689
Roh E, Song DK, Kim MS (2016) Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med 48:e216
Roth J, Harre EM, Rummel C, Gerstberger R, Hubschle T (2004) Signaling the brain in systemic inflammation: role of sensory circumventricular organs. Front Biosci 9:290–300
Süß P, Hoffmann A, Rothe T, Ouyang Z, Baum W, Staszewski O, Schett G, Prinz M et al (2020) Chronic peripheral inflammation causes a region-specific myeloid response in the central nervous system. Cell Rep 30(12):4082–4095
Thaler JP, Choi SJ, Schwartz MW, Wisse BE (2010) Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol 31:79–84
Borges BC, Garcia-Galiano D, Rorato R, Elias LLK, Elias CF (2016) PI3K p110 beta subunit in leptin receptor expressing cells is required for the acute hypophagia induced by endotoxemia. Mol Metab 5:379–391
Lee CH, Suk K, Yu R, Kim MS (2020) Cellular contributors to hypothalamic inflammation in obesity. Mol Cells 43:431–437
Seong J, Kang JY, Sun JS, Kim KW (2019) Hypothalamic inflammation and obesity: a mechanistic review. Arch Pharm Res 42:383–392
Jais A, Bruning JC (2017) Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest 127:24–32
Timper K, Paeger L, Sánchez-Lasheras C, Varela L, Jais A, Nolte H, Vogt MC, Hausen AC et al (2018) Mild impairment of mitochondrial OXPHOS promotes fatty acid utilization in POMC neurons and improves glucose homeostasis in obesity. Cell Rep 25(2):383-397 e10
Leloup C, Magnan C, Benani A, Bonnet E, Alquier T, Offer G, Carriere A, Périquet A et al (2006) Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes 55(7):2084–2090
Drougard A, Fournel A, Valet P, Knauf C (2015) Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake. Front Neurosci 9:56
Dietrich MO, Liu ZW, Horvath TL (2013) Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155:188–199
Santoro A, Campolo M, Liu C, Sesaki H, Meli R, Liu ZW, Kim JD, Diano S (2017) DRP1 suppresses leptin and glucose sensing of POMC neurons. Cell Metab 25(3):647–660
Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, Garcia A, Esteban Y, Gonzalez-Franquesa A et al (2013) Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155(1):172–187
Ramírez S, Gómez-Valadés AG, Schneeberger M, Varela L, Haddad-Tóvolli R, Altirriba J, Noguera E, Drougard A et al (2017) Mitochondrial dynamics mediated by mitofusin 1 is required for POMC neuron glucose-sensing and insulin release control. Cell Metab 25(6):1390-1399 e6
Michael NJ, Simonds SE, van den Top M, Cowley MA, Spanswick D (2017) Mitochondrial uncoupling in the melanocortin system differentially regulates NPY and POMC neurons to promote weight-loss. Mol Metab 6:1103–1112
Vezzani A, Viviani B (2015) Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacol 96:70–82
Rizzo FR, Musella A, De Vito F, Fresegna D, Bullitta S, Vanni V, Guadalupi L, StampanoniBassi M et al (2018) Tumor necrosis factor and interleukin-1 beta modulate synaptic plasticity during neuroinflammation. Neural Plast 2018:8430123
Park SY, Kang MJ, Han JS (2018) Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells. Mol Brain 11:39
Storer MA, Gallagher D, Fatt MP, Simonetta JV, Kaplan DR, Miller FD (2018) Interleukin-6 regulates adult neural stem cell numbers during normal and abnormal post-natal development. Stem Cell Reports 10(5):1464–1480
Erta M, Quintana A, Hidalgo J (2012) Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8:1254–1266
Gruol DL (2015) IL-6 regulation of synaptic function in the CNS. Neuropharmacology 96:42–54
Weischenfeldt J, Porse B (2008) Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc 2008:pdb prot5080
Nazarians-Armavil A, Chalmers JA, Lee CB, Ye W, Belsham DD (2014) Cellular insulin resistance disrupts hypothalamic mHypoA-POMC/GFP neuronal signaling pathways. J Endocrinol 220:13–24
Varela L, Horvath TL (2012) Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep 13:1079–1086
Blanque R, Meakin C, Millet S, Gardner CR (1998) Selective enhancement of LPS-induced serum TNF-alpha production by carrageenan pretreatment in mice. Gen Pharmacol 31:301–306
Dalvi PS, Chalmers JA, Luo V, Han DY, Wellhauser L, Liu Y, Tran DQ, Castel J et al (2017) High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-alpha on appetite-regulating NPY neurons. Int J Obes (Lond) 41(1):149–158
Liu F, Zhang G, Sheng X, Liu S, Cui M, Guo H, Xue J, Zhang L (2018) Effects of hereditary moderate high fat diet on metabolic performance and physical endurance capacity in C57BL/6 offspring. Mol Med Rep 17(3):4672–4680
Zhao RZ, Jiang S, Zhang L, Yu ZB (2019) Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med 44:3–15
Busiello RA, Savarese S, Lombardi A (2015) Mitochondrial uncoupling proteins and energy metabolism. Front Physiol 6:36
Chouchani ET, Kazak L, Spiegelman BM (2019) New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab 29:27–37
Villarroya F, Peyrou M, Giralt M (2017) Transcriptional regulation of the uncoupling protein-1 gene. Biochimie 134:86–92
Degan D, Ornello R, Tiseo C, Carolei A, Sacco S, Pistoia F (2018) The role of inflammation in neurological disorders. Curr Pharm Des 24(14):1485–1501
Jeon SW, Yoon HK, Kim YK (2019) Role of inflammation in psychiatric disorders. Adv Exp Med Biol 1192:491–501
Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17:49–59
Choi SS, Lee HJ, Lim I, Satoh J, Kim SU (2014) Human astrocytes: secretome profiles of cytokines and chemokines. PLoS ONE 9(4):e92325
Chitnis T, Weiner HL (2017) CNS inflammation and neurodegeneration. J Clin Invest 127:3577–3587
Tang YY, Holzel BK, Posner MI (2015) The neuroscience of mindfulness meditation. Nat Rev Neurosci 16:213–225
Lumeng CN, Saltiel AR (2011) Inflammatory links between obesity and metabolic disease. J Clin Invest 121:2111–2117
Cai D, Liu T (2011) Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann N Y Acad Sci 1243:E1-39
De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA (2005) Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinol 146(10):4192–4199
Jung CH, Kim MS (2013) Molecular mechanisms of central leptin resistance in obesity. Arch Pharm Res 36:201–207
Lee I, Huttemann M (2014) Energy crisis: the role of oxidative phosphorylation in acute inflammation and sepsis. Biochim Biophys Acta 1842:1579–1586
Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA (2004) Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res 64:279–288
Zuo H, Wan Y (2019) Metabolic reprogramming in mitochondria of myeloid cells. Cells 9(1):5
Doll DN, Rellick SL, Barr TL, Ren X, Simpkins JW (2015) Rapid mitochondrial dysfunction mediates TNF-alpha-induced neurotoxicity. J Neurochem 132:443–451
Russell AE, Doll DN, Sarkar SN, Simpkins JW (2016) TNF-alpha and beyond: rapid mitochondrial dysfunction mediates TNF-alpha-induced neurotoxicity. J Clin Cell Immunol 7(6):467
Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR et al (2007) Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449(7159):228–232
Diano S, Horvath TL (2012) Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med 18:52–58
Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschöp MH, Shanabrough M et al (2008) UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454(7206):846–851
Acknowledgements
We would like to acknowledge Mr. Clement Lee for his excellent technical support for TEM at Taipei Medical University Core Facility.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 106–2320-B-004–001).
Author information
Authors and Affiliations
Contributions
YCL, YSL, and PWC performed the experiments, analyzed data, prepared figures, and participated in manuscript writing; SKC designed experiments, integrated data, supervised, and wrote and reviewed the manuscript. All authors approved the final submission.
Corresponding author
Ethics declarations
Ethics Approval
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of National Chengchi University. All of the animal experiments were performed in compliance with the IACUC regulations and Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, YC., Lim, Y., Chu, PW. et al. Inflammatory Milieu Induces Mitochondrial Alterations and Neuronal Activations in Hypothalamic POMC Neurons in a Time-Dependent Manner. Mol Neurobiol 60, 1164–1178 (2023). https://doi.org/10.1007/s12035-022-03128-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03128-3