Abstract
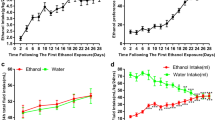
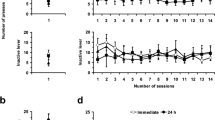
Alcohol use disorder (AUD) is a common and complex disorder resulting from repetitive alcohol drinking. The mesocorticolimbic dopamine (DA) system, originating from the ventral tegmental area (VTA) in the midbrain, is involved in the rewarding effect of ethanol. The γ-aminobutyric acid (GABA) neurons in VTA appear to be key substrates of acute and chronic ethanol, which regulates DA neurotransmission indirectly in the mesocorticolimbic system. Despite significant research on the relationship between brain-derived neurotrophic factor (BDNF) and reduced alcohol consumption in male rats involving tropomyosin-related kinase B (TrkB), the mechanisms of BDNF-TrkB regulating alcohol behavior remain scarce. K+-Cl– cotransporter 2 (KCC2) plays a crucial role in synaptic function in GABAergic neurons by modulating intracellular chlorine homeostasis. Here, we found that 4-week intermittent alcohol exposure impaired the function of KCC2 in VTA, evidenced by a lower expression level of phosphorylated KCC2 and decreased ratio of phosphorylated KCC2 to total KCC2, especially 72 h after withdrawal from 4-week ethanol exposure in male rats. CLP290 (a KCC2 activator) reduced excessive alcohol consumption after alcohol withdrawal, whereas VU0240551 (a specific KCC2 inhibitor) further enhanced alcohol intake. Importantly, VU0240551 reversed the attenuating effects of BDNF and 7,8-dihydroxyflavone (7,8-DHF) on alcohol consumption after withdrawal. Moreover, intraperitoneal injection of 7,8-DHF upregulated KCC2 expression and phosphorylated KCC2 in VTA 72 h after withdrawal from ethanol exposure in male rats. Collectively, our data indicate that KCC2 may be critical in the regulating action of BDNF-TrkB on ethanol consumption in AUD.




Similar content being viewed by others
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, Schmidt D, Watkins LR, Maier SF, et al. (2018) Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav Immun 73:352–363. https://doi.org/10.1016/j.bbi.2018.05.020
Siomek-Gorecka A, Dlugosz A, Czarnecki D (2021) The Molecular Basis of Alcohol Use Disorder (AUD). Genetics, epigenetics, and nutrition in AUD: an amazing triangle. Intern ational journal of molecular sciences, 22(8), 4262. https://doi.org/10.3390/ijms22084262
Castillo-Carniglia A, Keyes KM, Hasin DS, Cerdá M (2019) Psychiatric comorbidities in alcohol use disorder. The lancet Psychiatry 6(12):1068–1080. https://doi.org/10.1016/S2215-0366(19)30222-6
Nelson AC, Williams SB, Pistorius SS, Park HJ, Woodward TJ, Payne AJ, Obray JD, Shin SI, et al. (2018) Ventral tegmental area GABA neurons are resistant to GABA(A) receptor-mediated inhibition during ethanol withdrawal. Front Neurosci 12:131. https://doi.org/10.3389/fnins.2018.00131
Juarez B, Han MH (2016) Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharmacology 41(10):2424–2446. https://doi.org/10.1038/npp.2016.32
Ludlow KH, Bradley KD, Allison DW, Taylor SR, Yorgason JT, Hansen DM, Walton CH, Sudweeks SN, et al. (2009) Acute and chronic ethanol modulate dopamine D2-subtype receptor responses in ventral tegmental area GABA neurons. Alcohol Clin Exp Res 33(5):804–811. https://doi.org/10.1111/j.1530-0277.2009.00899.x
Chamma I, Chevy Q, Poncer JC, Lévi S (2012) Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front Cell Neurosci 6:5. https://doi.org/10.3389/fncel.2012.00005
Tillman L, Zhang J (2019) Crossing the chloride channel: the current and potential therapeutic value of the neuronal K+-Cl- cotransporter KCC2. Biomed Res Int 2019:8941046. https://doi.org/10.1155/2019/8941046
Fiumelli H, Woodin MA (2007) Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol 17(1):81–86. https://doi.org/10.1016/j.conb.2007.01.002
Guan YZ, Ye JH (2010) Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving mu-opioid receptors. Neuropsychopharmacology 35(9):1841–1849. https://doi.org/10.1038/npp.2010.51
Barde Y A (1994) Neurotrophins: a family of proteins supporting the survival of neurons. Progress in clinical and biological research, 390, 45–56.https://pubmed.ncbi.nlm.nih.gov/7724649/
Levine ES, Kolb JE (2000) Brain-derived neurotrophic factor increases activity of NR2B-containing N-methyl-D-aspartate receptors in excised patches from hippocampal neurons. J Neurosci Res 62(3):357–362. https://doi.org/10.1002/1097-4547(20001101)62:3%3c357::AID-JNR5%3e3.0.CO;2-6
Cheng Q, Yeh HH (2003) Brain-derived neurotrophic factor attenuates mouse cerebellar granule cell GABA(A) receptor-mediated responses via postsynaptic mechanisms. J Physiol 548(Pt 3):711–721. https://doi.org/10.1113/jphysiol.2002.037846
Wardle RA, Poo MM (2003) Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci 23(25):8722–8732. https://doi.org/10.1523/JNEUROSCI.23-25-08722.2003
Li XX, Yang T, Wang N, Zhang LL, Liu X, Xu YM, Gao Q, Zhu XF, et al. (2020) 7,8-Dihydroxyflavone attenuates alcohol-related behavior in rat models of alcohol consumption via TrkB in the ventral tegmental area. Front Neurosci 14:467. https://doi.org/10.3389/fnins.2020.00467
Ferrini F, De Koninck Y (2013) Microglia control neuronal network excitability via BDNF signalling. Neural Plast 2013:429815. https://doi.org/10.1155/2013/429815
Lee-Hotta S, Uchiyama Y, Kametaka S (2019) Role of the BDNF-TrkB pathway in KCC2 regulation and rehabilitatin following neuronal injury: a mini review. Neurochem Int 128:32–38. https://doi.org/10.1016/j.neuint.2019.04.003
Medina I, Friedel P, Rivera C, Kahle KT, Kourdougli N, Uvarov P, Pellegrino C (2014) Current view on the functional regulation of the neuronal K(+)-Cl(-) cotransporter KCC2. Front Cell Neurosci 8:27. https://doi.org/10.3389/fncel.2014.00027
Silvestre de Ferron B, Vilpoux C, Kervern M, Robert A, Antol J, Naassila M, Pierrefiche O (2017) Increase of KCC2 in hippocampal synaptic plasticity disturbances after perinatal ethanol exposure. Addict Biol 22(6):1870–1882. https://doi.org/10.1111/adb.12465
Santos L, Rodrigues AM, Lopes MR, Costa V, Scorza CA, Scorza FA, Cavalheiro EA, et al. (2017) Long-term alcohol exposure elicits hippocampal nonsynaptic epileptiform activity changes associated with expression and functional changes in NKCC1, KCC2 co-transporters and Na+/K+-ATPase. Neuroscience 340:530–541. https://doi.org/10.1016/j.neuroscience.2016.11.015
Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA (2016) Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron 92(2):493–504. https://doi.org/10.1016/j.neuron.2016.09.029
Kimmey B A, Ostroumov A, Dani J A (2019) 5-HT2A receptor activation normalizes stress-induced dysregulation of GABAergic signaling in the ventral tegmental area. Proceedings of the National Academy of Sciences of the United States of America, 116(52), 27028–27034. Advance online publication. https://doi.org/10.1073/pnas.1911446116
Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T, Dani JA (2018) Adolescent nicotine exposure alters GABAA receptor signaling in the ventral tegmental area and increases adult ethanol self-administration. Cell Rep 23(1):68–77. https://doi.org/10.1016/j.celrep.2018.03.030
Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y (2004) A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci 24(7):1604–1611. https://doi.org/10.1523/JNEUROSCI.5124-03.2004
Ye JH, Zhang J, Xiao C, Kong JQ (2006) Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods 158(2):251–259. https://doi.org/10.1016/j.jneumeth.2006.06.006
Deisz RA, Wierschke S, Schneider UC, Dehnicke C (2014) Effects of VU0240551, a novel KCC2 antagonist, and DIDS on chloride homeostasis of neocortical neurons from rats and humans. Neuroscience 277:831–841. https://doi.org/10.1016/j.neuroscience.2014.07.037
Paxinos, G., Watson, C., 2006. The rat brain. In: Stereotaxic Coordinates, 6th ed. eBook.
Kahle KT, Deeb TZ, Puskarjov M, Silayeva L, Liang B, Kaila K, Moss SJ (2013) Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci 36(12):726–737. https://doi.org/10.1016/j.tins.2013.08.006
Haun HL, Griffin WC, Lopez MF, Solomon MG, Mulholland PJ, Woodward JJ, McGinty JF, Ron D, et al. (2018) Increasing Brain-Derived Neurotrophic Factor (BDNF) in medial prefrontal cortex selectively reduces excessive drinking in ethanol dependent mice. Neuropharmacology 140:35–42. https://doi.org/10.1016/j.neuropharm.2018.07.031
Wang N, Liu X, Li XT, Li XX, Ma W, Xu YM, Liu Y, Gao Q, et al. (2021) 7,8-Dihydroxyflavone alleviates anxiety-like behavior induced by chronic alcohol exposure in mice involving tropomyosin-related kinase B in the amygdala. Mol Neurobiol 58(1):92–105. https://doi.org/10.1007/s12035-020-02111-0
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35(1):217–238. https://doi.org/10.1038/npp.2009.110
Zhou Y, Kreek MJ (2021) Blockade of alcohol excessive and “relapse” drinking in male mice by pharmacological cryptochrome (CRY) activation. Psychopharmacology 238(4):1099–1109. https://doi.org/10.1007/s00213-020-05757-9
Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB (2008) Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol 4(9):490–503. https://doi.org/10.1038/ncpneuro0883
Taylor AM, Castonguay A, Ghogha A, Vayssiere P, Pradhan AA, Xue L, Mehrabani S, Wu J, et al. (2016) Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology 41(4):949–959. https://doi.org/10.1038/npp.2015.221
Uhl GR, Koob GF, Cable J (2019) The neurobiology of addiction. Ann N Y Acad Sci 1451(1):5–28. https://doi.org/10.1111/nyas.13989
Shibasaki M, Tsuyuki T, Ando K, Otokozawa A, Udagawa Y, Watanabe K, Shibasaki Y, Mori T, et al. (2014) Implication of KCC2 in the sensitization to morphine by chronic ethanol treatment in mice. Synapse (New York, N.Y.), 68(1), 39–43. https://doi.org/10.1002/syn.21688
Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J (2014) Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15(10):637–654. https://doi.org/10.1038/nrn3819
Lee HH, Walker JA, Williams JR, Goodier RJ, Payne JA, Moss SJ (2007) Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J Biol Chem 282(41):29777–29784. https://doi.org/10.1074/jbc.M705053200
Stubbs C D., Slater S J.(1999). Ethanol and protein kinase C. Alcohol Clin Exp Res, 23(9), 1552–60. https://doi.org/10.1111/j.1530-0277.1999.tb04680.x
Jiang ZL, Ye JH (2003) Protein kinase C epsilon is involved in ethanol potentiation of glycine-gated Cl(-) current in rat neurons of ventral tegmental area. Neuropharmacology 44(4):493–502. https://doi.org/10.1016/s0028-3908(02)00409-4
Watanabe M, Fukuda A (2015) Development and regulation of chloride homeostasis in the central nervous system. Front Cell Neurosci 9:371. https://doi.org/10.3389/fncel.2015.00371
Koponen E, Võikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, Taira T, Castrén E (2004) Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci 26(1):166–181. https://doi.org/10.1016/j.mcn.2004.01.006
Franke TF, Kaplan DR, Cantley LC (1997) PI3K: downstream AKTion blocks apoptosis. Cell 88(4):435–437. https://doi.org/10.1016/s0092-8674(00)81883-8
Seger R, Krebs EG (1995) The MAPK signaling cascade. FASEB J 9(9):726–735. https://doi.org/10.1523/JNEUROSCI.5265-03.2004
Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipilä S, Payne JA, Minichiello L, et al. (2004) Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci 24(19):4683–4691. https://doi.org/10.1523/JNEUROSCI.5265-03.2004
Hyman SE (2007) How mice cope with stressful social situations. Cell 131(2):232–234. https://doi.org/10.1016/j.cell.2007.10.008
Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2(2):119–128. https://doi.org/10.1038/35053570
Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G (1985) Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res 348(1):201–203. https://doi.org/10.1016/0006-8993(85)90381-6
Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology 205(4):529–564. https://doi.org/10.1007/s00213-009-1562-z
Mercado A, Mount DB, Gamba G (2004) Electroneutral cation-chloride cotransporters in the central nervous system. Neurochem Res 29(1):17–25. https://doi.org/10.1023/b:nere.0000010432.44566.21
Payne JA, Rivera C, Voipio J, Kaila K (2003) Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci 26(4):199–206. https://doi.org/10.1016/S0166-2236(03)00068-7
Gulyás AI, Sík A, Payne JA, Kaila K, Freund TF (2001) The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur J Neurosci 13(12):2205–2217. https://doi.org/10.1046/j.0953-816x.2001.01600.x
Di Cristo G, Awad PN, Hamidi S, Avoli M (2018) KCC2, epileptiform synchronization, and epileptic disorders. Prog Neurobiol 162:1–16. https://doi.org/10.1016/j.pneurobio.2017.11.002
Vandegrift BJ, You C, Satta R, Brodie MS, Lasek AW (2017) Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS ONE 12:e0187698. https://doi.org/10.1371/journal.pone.0187698
Acknowledgements
This study was supported by the National Science Foundation of China (NSFC) (81871041 to Y.Z.G.), Key Project of Heilongjiang Natural Science Foundation (ZD2022H007), Fundamental Scientific Research Project of Colleges in Heilongjiang Province (2021-KYYWF-0478), Key R & D Plan of Heilongjiang Province (GA21C010 to X.F.Z.), and Graduate Innovative Research Programs of Mudanjiang Medical University, China (2020YJSCX-MY03, Y.Z.G.).
Funding
This study was supported by the National Science Foundation of China (NSFC) (81871041 to Y.Z.G.), Key Project of Heilongjiang Natural Science Foundation (ZD2022H007 to Y.Z.G.), Fundamental Scientific Research Project of Colleges in Heilongjiang Province (2021-KYYWF-0478), Key R & D Plan of Heilongjiang Province (GA21C010 to X.F.Z.), and Graduate Innovative Research Programs of Mudanjiang Medical University, China (2020YJSCX-MY03, Y.Z.G.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yanzhong Guan conceived the project. Material preparation, data collection, and analysis were performed by Hongyan Zhang, Lulu Xu, Junwei Xiong, Xinxin Li, Yindong Yang, Yong Liu, Chunfeng Zhang, Qiyu Wang, Jiajia Wang, Pengyu Wang, Xiaobin Wu, Xue Wang, and Xiaofeng Zhu. The first draft of the manuscript was written by Hongyan Zhang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Research Involving Human Participants and/or Animals
All procedures were performed in accordance with the guidelines in the National Institutes of Health Guide for Care and Use of Laboratory Animals, and the study design was approved by the appropriate ethics review board.
Consent for Publication
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. All authors read and approved the final manuscript. The authors consent for publication in Molecular Neurobiology.
Consent to participate
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Xu, L., Xiong, J. et al. Role of KCC2 in the Regulation of Brain-Derived Neurotrophic Factor on Ethanol Consumption in Rats. Mol Neurobiol 60, 1040–1049 (2023). https://doi.org/10.1007/s12035-022-03126-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03126-5