Abstract
Failed communication between mitochondria and lysosomes causes dysfunctional mitochondria, which may induce mitochondria-related neurodegenerative diseases. Here, we show that RAB7A, a small GTPase of the Rab family, mediates the crosstalk between these two important organelles to maintain homeostasis in N2a cells treated with PrP106–126. Specifically, we demonstrate that mitophagy deficiency in N2a cells caused by PrP106–126 is associated with dysregulated RAB7A localization in mitochondria. Cells lacking RAB7A display decreased mitochondrial colocalization with lysosomes and significantly increased mitochondrial protein expression, resulting in inhibited mitophagy. In contrast, overexpression of GTP-bound RAB7A directly induces lysosome colocalization with mitochondria. Further study revealed that GTP-bound RAB7A protects mitochondrial homeostasis by supporting autophagosome biogenesis. Moreover, we suggest that depletion of RAB7A leads to gross morphological changes in lysosomes, which prevents autophagosome–lysosome fusion and interferes with the breakdown of autophagic cargo within lysosomes. Overexpression of GTP-bound RAB7A can also alleviate PrP106–126-induced morphological damage and dysfunction of mitochondria, reducing neuronal apoptosis. Collectively, our data demonstrate that RAB7A successfully drives mitochondria to the autophagosomal lumen for degradation, suggesting that the communication of proteotoxic stress from mitochondria to lysosomes requires RAB7A, as a signaling molecule, to establish a link between the disturbed mitochondrial network and its remodeling. These findings indicate that small molecules regulating mitophagy have the potential to modulate cellular homeostasis and the clinical course of neurodegenerative diseases.
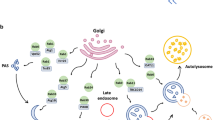
Graphical Abstract
Proposed model of mitophagy regulated by RAB7A. (1) Accumulating PrP106–126 induced mitophagy. (2) RAB7A is recruited to mitochondria. (3) ATG5-12 and ATG9A (5) vesicles are recruited to the autophagosome formation sites in a RAB7A-dependent manner. The ATG5-12 complex recruits and anchors LC3-I to form active LC3-II (4), accelerating mitophagosomal formation. The ATG9A vesicles are thought to be a source of membranes for autophagosome assembly. The recruitment of proteins and lipids induces membrane expansion and subsequent closure to form the mitophagosome. (6) Maintenance of the normal low lysosomal PH depends on active (GTP-bound) RAB7A. (7) RAB7A recruits effector molecules responsible for tight membrane interactions, and directly or indirectly, the subsequent autophagosome merges with the lysosome, and the cargo is completely degraded.








Similar content being viewed by others
Data Availability
Most of the data generated or analyzed during this study are included in the published article and its supplementary information files. Additional generated datasets are available from the corresponding author on reasonable request.
References
Forloni G, Chiesa R, Bugiani O, Salmona M, Tagliavini F (2019) Review: PrP 106–126 - 25 years after [J]. Neuropathol Appl Neurobiol 45(5):430–440
Wang XS, Zhang BB, Zhao C, Wang YL, He L, Cui MH et al (2013) Inhibition of human prion neuropeptide PrP106-126 aggregation by hexacoordinated ruthenium complexes [J]. J Inorg Biochem 128:1–10
Tagliavini F, Prelli F, Verga L, Giaccone G, Sarma R, Gorevic P et al (1993) Synthetic peptides homologous to prion protein residues 106–147 form amyloid-like fibrils in-vitro [J]. Proc Natl Acad Sci USA 90(20):9678–9682
Heegaard PMH, Pedersen HG, Flink J, Boas U (2004) Amyloid aggregates of the prion peptide PrP106-126 are destabilised by oxidation and by the action of dendrimers [J]. FEBS Lett 577(1–2):127–133
Kuwata K, Matumoto T, Cheng H, Nagayama K, James TL, Roder H (2003) NMR-detected hydrogen exchange and molecular dynamics simulations provide structural insight into fibril formation of prion protein fragment 106–126 [J]. Proc Natl Acad Sci USA 100(25):14790–14795
Fivenson EM, Lautrup S, Sun N, Scheibye-Knudsen M, Stevnsner T, Nilsen H et al (2017) Mitophagy in neurodegeneration and aging [J]. Neurochem Int 109:202–209
Yu L, Chen Y, Tooze SA (2018) Autophagy pathway: cellular and molecular mechanisms [J]. Autophagy 14(2):207–215
Mercer TJ, Gubas A, Tooze SA (2018) A molecular perspective of mammalian autophagosome biogenesis [J]. J Biol Chem 293(15):5386–5395
Wu W, Zhao DM, Shah SZA, Zhang XX, Lai MY, Yang DM et al (2019) OPA1 overexpression ameliorates mitochondrial cristae remodeling, mitochondrial dysfunction, and neuronal apoptosis in prion diseases [J]. Cell Death Dis 10(10):710
Li CS, Wang D, Wu W, Yang W, Shah SZA, Zhao Y et al (2018) DLP1-dependent mitochondrial fragmentation and redistribution mediate prion-associated mitochondrial dysfunction and neuronal death [J]. Aging Cell 17(1):e12693
Pan YQ, Sun LY, Wang JH, Fu WZ, Fu YY, Wang J et al (2015) STI571 protects neuronal cells from neurotoxic prion protein fragment-induced apoptosis [J]. Neuropharmacology 93:191–198
Basson MA (2012) Signaling in cell differentiation and morphogenesis [J]. Csh Perspect Biol 4(6):a008151
Mima J (2018) Reconstitution of membrane tethering mediated by Rab-family small GTPases [J]. Biophys Rev 10(2):543–549
Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B (2000) Rab7: a key to lysosome biogenesis [J]. Mol Biol Cell 11(2):467–480
Progida C, Cogli L, Piro F, De Luca A, Bakke O, Bucci C (2010) Rab7b controls trafficking from endosomes to the TGN [J]. J Cell Sci 123(9):1480–1491
Progida C, Nielsen MS, Koster G, Bucci C, Bakke O (2012) Dynamics of Rab7b-Dependent Transport of Sorting Receptors [J]. Traffic 13(9):1273–1285
Mizuno-Yamasaki E, Rivera-Molina F, Novick P (2012) GTPase networks in membrane traffic [J]. Annu Rev Biochem 81(81):637–659
Gutierrez MG, Munafo DB, Beron W, Colombo MI (2004) Rab7 is required for the normal progression of the autophagic pathway in mammalian cells [J]. J Cell Sci 117(Pt 13):2687–2697
Hyttinen JMT, Niittykoski M, Salminen A, Kaarniranta K (2013) Maturation of autophagosomes and endosomes: a key role for Rab7 [J]. Bba-Mol Cell Res 1833(3):503–510
Zhao T, Huang X, Han L, Wang X, Cheng H, Zhao Y et al (2012) Central role of mitofusin 2 in autophagosome-lysosome fusion in cardiomyocytes [J]. J Biol Chem 287(28):23615–23625
Nakamura S, Yoshimori T (2017) New insights into autophagosome-lysosome fusion [J]. J Cell Sci 130(7):1209–1216
Guerra F, Bucci C (2016) Multiple roles of the small GTPase Rab7 [J]. Cells 5(3):34
Yamano K, Fogel AI, Wang C, van der Bliek AM, Youle RJ (2014) Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy [J]. Elife 3:e01612
Yamano K, Wang C, Sarraf SA, Munch C, Kikuchi R, Noda NN et al (2018) Endosomal Rab cycles regulate Parkin-mediated mitophagy [J]. Elife 7:e31326
Heo JM, Ordureau A, Swarup S, Paulo JA, Shen K, Sabatini DM et al (2018) RAB7A phosphorylation by TBK1 promotes mitophagy via the PINK-PARKIN pathway [J]. Sci Adv 4(11):eaav0443
Cogli L, Progida C, Thomas CL, Spencer-Dene B, Donno C, Schiavo G et al (2013) Charcot-Marie-Tooth type 2B disease-causing RAB7A mutant proteins show altered interaction with the neuronal intermediate filament peripherin [J]. Acta Neuropathol 125(2):257–272
Muller MP, Goody RS (2018) Molecular control of Rab activity by GEFs, GAPs and GDI [J]. Small GTPases 9(1–2):5–21
Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions [J]. Science 294(5545):1299–1304
Ham D, Ahn D, Ashim J, Cho Y, Kim HR, Yu W et al (2021) Conformational switch that induces GDP release from Gi [J]. J Struct Biol 213(1):107694
Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic [J]. Proc Natl Acad Sci 103(32):11821–11827
McCray BA, Skordalakes E, Taylor JP (2010) Disease mutations in Rab7 result in unregulated nucleotide exchange and inappropriate activation [J]. Hum Mol Genet 19(6):1033–1047
Jimenez-Orgaz A, Kvainickas A, Nagele H, Denner J, Eimer S, Dengjel J et al (2018) Control of RAB7 activity and localization through the retromer-TBC1D5 complex enables RAB7-dependent mitophagy [J]. EMBO J 37(2):235–254
Young ARJ, Chan EYW, Hu XW, Koch R, Crawshaw SG, High S et al (2006) Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes [J]. J Cell Sci 119(18):3888–3900
Orsi A, Razi M, Dooley HC, Robinson D, Weston AE, Collinson LM et al (2012) Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy [J]. Mol Biol Cell 23(10):1860–1873
Jin X, Wang K, Wang L, Liu W, Zhang C, Qiu Y et al (2021) RAB7 activity is required for the regulation of mitophagy in oocyte meiosis and oocyte quality control during ovarian aging [J]. Autophagy 18(3):643–660
Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology [J]. Physiol Rev 91(1):119–149
Zhen Y, Stenmark H (2015) Cellular functions of Rab GTPases at a glance [J]. J Cell Sci 128(17):3171–3176
Tong J, Tan L, Chun C, Im YJ (2019) Structural basis of human ORP1-Rab7 interaction for the late-endosome and lysosome targeting [J]. PLoS ONE 14(2):e0211724
Wong E, Cuervo AM (2010) Integration of clearance mechanisms: the proteasome and autophagy [J]. Cold Spring Harb Perspect Biol 2(12):a006734
Zhao YG, Codogno P, Zhang H (2021) Machinery, regulation and pathophysiological implications of autophagosome maturation [J]. Nat Rev Mol Cell Biol 22(11):733–750
Cai CZ, Zhuang XX, Zhu Q, Wu MY, Su H, Wang XJ et al (2022) Enhancing autophagy maturation with CCZ1-MON1A complex alleviates neuropathology and memory defects in Alzheimer disease models [J]. Theranostics 12(4):1738–1755
Sun N, Malide D, Liu J, Rovira II, Combs CA, Finkel T (2017) A fluorescence-based imaging method to measure in vitro and in vivo mitophagy using mt-Keima [J]. Nat Protoc 12(8):1576–1587
Nguyen TN, Padman BS, Usher J, Oorschot V, Ramm G, Lazarou M (2016) Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation [J]. J Cell Biol 215(6):857–874
Wang C (2020) A Sensitive and quantitative mKeima assay for mitophagy via FACS [J]. Curr Protoc Cell Biol 86(1):e99
Hoshino A, Wang WJ, Wada S, McDermott-Roe C, Evans CS, Gosis B et al (2019) The ADP/ATP translocase drives mitophagy independent of nucleotide exchange [J]. Nature 575(7782):375–379
Li J, Lai M, Zhang X, Li Z, Yang D, Zhao M et al (2022) PINK1-parkin-mediated neuronal mitophagy deficiency in prion disease [J]. Cell Death Dis 13(2):162
Audano M, Schneider A, Mitro N (2018) Mitochondria, lysosomes, and dysfunction: their meaning in neurodegeneration [J]. J Neurochem 147(3):291–309
Yambire KF, Rostosky C, Watanabe T, Pacheu-Grau D, Torres-Odio S, Sanchez-Guerrero A et al (2019) Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo [J]. Elife 8:e51031
Scarffe LA, Stevens DA, Dawson VL, Dawson TM (2014) Parkin and PINK1: much more than mitophagy [J]. Trends Neurosci 37(6):315–324
Nguyen TN, Padman BS, Lazarou M (2016) Deciphering the molecular signals of PINK1/Parkin mitophagy [J]. Trends Cell Biol 26(10):733–744
Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey CA, McNiven MA (2015) The small GTPase Rab7 as a central regulator of hepatocellular lipophagy [J]. Hepatology 61(6):1896–1907
De Luca M, Bucci C (2014) A new V-ATPase regulatory mechanism mediated by the Rab interacting lysosomal protein (RILP) [J]. Commun Integr Biol 7(5):e971572
Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J et al (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors [J]. Curr Biol 11(21):1680–1685
Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A et al (2010) FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport [J]. J Cell Biol 188(2):253–269
De Luca M, Cogli L, Progida C, Nisi V, Pascolutti R, Sigismund S et al (2014) RILP regulates vacuolar ATPase through interaction with the V1G1 subunit [J]. J Cell Sci 127(12):2697–2708
Sarkar C, Sadhukhan T, Bagh MB, Appu AP, Chandra G, Mondal A et al (2020) Cln1-mutations suppress Rab7-RILP interaction and impair autophagy contributing to neuropathology in a mouse model of infantile neuronal ceroid lipofuscinosis [J]. J Inherit Metab Dis 43(5):1082–1101
Lin Y, Wu CC, Wang XY, Kemper T, Squire A, Gunzer M et al (2019) Hepatitis B virus is degraded by autophagosome-lysosome fusion mediated by Rab7 and related components [J]. Protein Cell 10(1):60–66
Zhang XC, Li H (2019) Interplay between the electrostatic membrane potential and conformational changes in membrane proteins [J]. Protein Sci 28(3):502–512
Acknowledgements
We thank all the Prion Laboratory members for technical support and input. We are grateful to Prof. Qiao Jian and Lifeng Yang for helpful ideas and experimental design. We also thank Prof. Jianxin Chen of South China Agricultural University for manuscript revisions.
Funding
This work was supported by the Natural Science Foundation of China (Project No.31972641).
Author information
Authors and Affiliations
Contributions
ZL and LY conceived and designed the experiments. ZL performed the experiments, and ZL interpreted the results. ZL, JQ, and LY contributed to manuscript writing. LY reviewed and revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
This study did not require ethical approval.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Lai, M., Li, J. et al. RAB7A GTPase Is Involved in Mitophagosome Formation and Autophagosome–Lysosome Fusion in N2a Cells Treated with the Prion Protein Fragment 106–126. Mol Neurobiol 60, 1391–1407 (2023). https://doi.org/10.1007/s12035-022-03118-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03118-5