Abstract
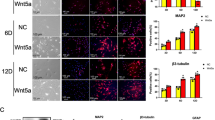
The therapeutic application of neural stem cells (NSCs) in the central nerve system (CNS) injury is a promising strategy for combating irreversible neuronal loss. However, a variety of obvious inflammatory responses following nerve injury rapidly create an unfavorable microenvironment for survival and neuronal differentiation of NSCs in lesion area, limiting the efficacy of NSC-based therapy for CNS injury. It remained unknown how to effectively increase the neuronal differentiation efficiency of NSCs through transplantation. Here, we demonstrated that curcumin (CCM)-activated olfactory ensheathing cells (aOECs) effectively promoted neuronal differentiation of NSCs in the activated microglial inflammatory condition, and co-transplantation of aOECs and NSCs improved neurological recovery of rats after spinal cord injury (SCI), as evidenced by higher expression levels of neuronal markers and lower expression levels of glial markers in the differentiated cells, greater number of Tuj-1-positive cells as well as higher Basso, Beattie, and Bresnahan (BBB) locomotor scale, compared to the corresponding controls. Pathologically, hematoxylin and eosin (HE) staining and immunostaining also showed that aOECs remarkably enhanced the in vivo neuronal differentiation of NSCs and migration, and nerve repair. Further analysis revealed that the underlying mechanisms of aOECs potentiating the neuronal conversion of NSCs under inflammatory environment were tightly associated with up-regulation of anti-inflammatory cytokines and neurotrophic factors in OECs, and importantly, the activation of Wnt3/β-catenin pathway was likely involved in the mechanisms underlying the observed cellular events. Therefore, this study provides a promising strategy for SCI repair by co-transplantation of aOECs and NSCs.










Similar content being viewed by others
Data availability
All data supporting the conclusions of this article are available from the corresponding author on reasonable request.
References
Badhiwala JH, Ahuja CS, Fehlings MG (2018) Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine 30(1):1–18. https://doi.org/10.3171/2018.9.SPINE18682
Dalamagkas K, Tsintou M, Seifalian AM (2018) Stem cells for spinal cord injuries bearing translational potential. Neural Regen Res 13(1):35–42. https://doi.org/10.4103/1673-5374.224360
Grabher BJ (2018) Effects of Alzheimer Disease on Patients and Their Family. J Nucl Med Technol 46(4):335–340. https://doi.org/10.2967/jnmt.118.218057
Yang B, Zhang F, Cheng F, Ying L, Wang C, Shi K, Wang J, Xia K et al (2020) Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis 11(6):439. https://doi.org/10.1038/s41419-020-2620-z
Harris LD, Jasem S, Licchesi J (2020) The Ubiquitin System in Alzheimer’s Disease. Adv Exp Med Biol 1233:195–221. https://doi.org/10.1007/978-3-030-38266-7_8
Ahuja CS, Wilson JR, Nori S, Kotter M, Druschel C, Curt A, Fehlings MG (2017) Traumatic spinal cord injury. Nat Rev Dis Primers 3:17018. https://doi.org/10.1038/nrdp.2017.18
Yip PK, Malaspina A (2012) Spinal cord trauma and the molecular point of no return. Mol Neurodegener 7:6. https://doi.org/10.1186/1750-1326-7-6
Norenberg MD, Smith J, Marcillo A (2004) The pathology of human spinal cord injury: defining the problems. J Neurotrauma 21(4):429–440. https://doi.org/10.1089/089771504323004575
Chi H, Chang HY, Sang TK (2018) Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci 19(10): 3082. https://doi.org/10.3390/ijms19103082
Maniatis S, Aijo T, Vickovic S, Braine C, Kang K, Mollbrink A, Fagegaltier D, Andrusivova Z et al (2019) Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 364(6435):89–93. https://doi.org/10.1126/science.aav9776
Hsu JM, Kang Y, Corty MM, Mathieson D, Peters OM, Freeman MR (2021) Injury-Induced Inhibition of Bystander Neurons Requires dSarm and Signaling from Glia. Neuron 109(3):473–487. https://doi.org/10.1016/j.neuron.2020.11.012
Xie J, Li Y, Dai J, He Y, Sun D, Dai C, Xu H, Yin ZQ (2019) Olfactory Ensheathing Cells Grafted Into the Retina of RCS Rats Suppress Inflammation by Down-Regulating the JAK/STAT Pathway. Front Cell Neurosci 13:341. https://doi.org/10.3389/fncel.2019.00341
Doi D, Magotani H, Kikuchi T, Ikeda M, Hiramatsu S, Yoshida K, Amano N, Nomura M et al (2020) Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat Commun 11(1):3369. https://doi.org/10.1038/s41467-020-17165-w
Grochowski C, Radzikowska E, Maciejewski R (2018) Neural stem cell therapy-Brief review. Clin Neurol Neurosurg 173:8–14. https://doi.org/10.1016/j.clineuro.2018.07.013
Zhai W, Gao L, Qu L, Li Y, Zeng Y, Li Q, Xu H, Yin ZQ (2020) Combined Transplantation of Olfactory Ensheathing Cells With Rat Neural Stem Cells Enhanced the Therapeutic Effect in the Retina of RCS Rats. Front Cell Neurosci 14:52. https://doi.org/10.3389/fncel.2020.00052
Wang X, Kuang N, Chen Y, Liu G, Wang N, Kong F, Yue S, Zheng Z (2021) Transplantation of olfactory ensheathing cells promotes the therapeutic effect of neural stem cells on spinal cord injury by inhibiting necrioptosis. Aging Albany NY 13(6):9056–9070. https://doi.org/10.18632/aging.202758
Luo ML, Pan L, Wang L, Wang HY, Li S, Long ZY, Zeng L, Liu Y (2019) Transplantation of NSCs Promotes the Recovery of Cognitive Functions by Regulating Neurotransmitters in Rats with Traumatic Brain Injury. Neurochem Res 44(12):2765–2775. https://doi.org/10.1007/s11064-019-02897-z
Yang Y, Fan Y, Zhang H, Zhang Q, Zhao Y, Xiao Z, Liu W, Chen B et al (2021) Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials 269:120479. https://doi.org/10.1016/j.biomaterials.2020.120479
Zhu Q, Zhang N, Hu N, Jiang R, Lu H, Xuan A, Long D, Chen Y (2020) Neural stem cell transplantation improves learning and memory by protecting cholinergic neurons and restoring synaptic impairment in an amyloid precursor protein/presenilin 1 transgenic mouse model of Alzheimer’s disease. Mol Med Rep 21(3):1172–1180. https://doi.org/10.3892/mmr.2020.10918
Cheng Z, Zhu W, Cao K, Wu F, Li J, Wang G, Li H, Lu M et al (2016) Anti-inflammatory mechanism of neural stem cell transplantation in spinal cord injury. Int J Mol Sci 17(9):1380. https://doi.org/10.3390/ijms17091380
Ricci-Vitiani L, Casalbore P, Petrucci G, Lauretti L, Montano N, Larocca LM, Falchetti ML, Lombardi DG et al (2006) Influence of local environment on the differentiation of neural stem cells engrafted onto the injured spinal cord. Neurol Res 28(5):488–492. https://doi.org/10.1179/016164106X115134
Coutts M, Keirstead HS (2008) Stem cells for the treatment of spinal cord injury. Exp Neurol 209(2):368–377. https://doi.org/10.1016/j.expneurol.2007.09.002
Fan L, Liu C, Chen X, Zou Y, Zhou Z, Lin C, Tan G, Zhou L et al (2018) Directing Induced Pluripotent Stem Cell Derived Neural Stem Cell Fate with a Three-Dimensional Biomimetic Hydrogel for Spinal Cord Injury Repair. ACS Appl Mater Interfaces 10(21):17742–17755. https://doi.org/10.1021/acsami.8b05293
Selvaraj V, Jiang P, Chechneva O, Lo UG, Deng W (2012) Differentiating human stem cells into neurons and glial cells for neural repair. Front Biosci (Landmark Ed) 17(1):65–89. https://doi.org/10.2741/3916
Li Y, Huo S, Fang Y, Zou T, Gu X, Tao Q, Xu H (2018) ROCK Inhibitor Y27632 Induced Morphological Shift and Enhanced Neurite Outgrowth-Promoting Property of Olfactory Ensheathing Cells via YAP-Dependent Up-Regulation of L1-CAM. Front Cell Neurosci 12:489. https://doi.org/10.3389/fncel.2018.00489
Zhang L, Zhuang X, Kotitalo P, Keller T, Krzyczmonik A, Haaparanta-Solin M, Solin O, Forsback S et al (2021) Intravenous transplantation of olfactory ensheathing cells reduces neuroinflammation after spinal cord injury via interleukin-1 receptor antagonist. Theranostics 11(3):1147–1161. https://doi.org/10.7150/thno.52197
Giordano A, Tommonaro G (2019) Curcumin and cancer. Nutrients 11(10):2376. https://doi.org/10.3390/nu11102376
Tomeh MA, Hadianamrei R, Zhao X (2019) A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci 20(5):1033. https://doi.org/10.3390/ijms20051033
Tsuda T (2018) Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct 9(2):705–714. https://doi.org/10.1039/c7fo01242j
Hao DJ, Liu C, Zhang L, Chen B, Zhang Q, Zhang R, An J, Zhao J et al (2017) Lipopolysaccharide and Curcumin Co-Stimulation Potentiates Olfactory Ensheathing Cell Phagocytosis Via Enhancing Their Activation. Neurotherapeutics 14(2):502–518. https://doi.org/10.1007/s13311-016-0485-8
Guo J, Cao G, Yang G, Zhang Y, Wang Y, Song W, Xu Y, Ma T et al (2020) Transplantation of activated olfactory ensheathing cells by curcumin strengthens regeneration and recovery of function after spinal cord injury in rats. Cytotherapy 22(6):301–312. https://doi.org/10.1016/j.jcyt.2020.03.002
He BR, Xie ST, Wu MM, Hao DJ, Yang H (2014) Phagocytic removal of neuronal debris by olfactory ensheathing cells enhances neuronal survival and neurite outgrowth via p38MAPK activity. Mol Neurobiol 49(3):1501–1512. https://doi.org/10.1007/s12035-013-8588-2
Koss K, Churchward MA, Tsui C, Todd KG (2019) In Vitro Priming and Hyper-Activation of Brain Microglia: an Assessment of Phenotypes. Mol Neurobiol 56(9):6409–6425. https://doi.org/10.1007/s12035-019-1529-y
Pulido-Salgado M, Vidal-Taboada JM, Barriga GG, Sola C, Saura J (2018) RNA-Seq transcriptomic profiling of primary murine microglia treated with LPS or LPS + IFNgamma. Sci Rep 8(1):16096. https://doi.org/10.1038/s41598-018-34412-9
Zhang X, Zhu XL, Ji BY, Cao X, Yu LJ, Zhang Y, Bao XY, Xu Y et al (2019) LncRNA-1810034E14Rik reduces microglia activation in experimental ischemic stroke. J Neuroinflammation 16(1):75. https://doi.org/10.1186/s12974-019-1464-x
Yang H, Liu C, Fan H, Chen B, Huang D, Zhang L, Zhang Q, An J et al (2019) Sonic Hedgehog Effectively Improves Oct4-Mediated Reprogramming of Astrocytes into Neural Stem Cells. Mol Ther 27(8):1467–1482. https://doi.org/10.1016/j.ymthe.2019.05.006
Kong G, Huang Z, Zhu Q, Wan Y (2020) Comparison of two modified methods of intrathecal catheterization in rats. Exp Anim 69(2):219–223. https://doi.org/10.1538/expanim.19-0108
Yang W, Sun P (2020) Promoting functions of microRNA-29a/199B in neurological recovery in rats with spinal cord injury through inhibition of the RGMA/STAT3 axis. J Orthop Surg Res 15(1):427. https://doi.org/10.1186/s13018-020-01956-4
Zhou Q, Guo D, Li X, Wang Y, Ye X, Xue S, Wang X (2020) Anti-inflammatory effects of vinpocetine in LPS-stimulated microglia via activation of AMPK. An Acad Bras Cienc 92(4):e20200241. https://doi.org/10.1590/0001-3765202020200241
An J, Chen B, Kang X, Zhang R, Guo Y, Zhao J, Yang H (2020) Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am J Transl Res 12(6):2353–2378
Sharma M, Arbabzada N, Flood PM (2019) Mechanism underlying beta2-AR agonist-mediated phenotypic conversion of LPS-activated microglial cells. J Neuroimmunol 332:37–48. https://doi.org/10.1016/j.jneuroim.2019.03.017
Yu J, Guo M, Li Y, Zhang H, Chai Z, Wang Q, Yan Y, Yu J et al (2019) Astragaloside IV protects neurons from microglia-mediated cell damage through promoting microglia polarization. Folia Neuropathol 57(2):170–181. https://doi.org/10.5114/fn.2019.86299
Farhood B, Mortezaee K, Goradel NH, Khanlarkhani N, Salehi E, Nashtaei MS, Najafi M, Sahebkar A (2019) Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J Cell Physiol 234(5):5728–5740. https://doi.org/10.1002/jcp.27442
Wang Q, Ye C, Sun S, Li R, Shi X, Wang S, Zeng X, Kuang N et al (2019) Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int Immunopharmacol 72:292–300. https://doi.org/10.1016/j.intimp.2019.04.027
Orr MB, Gensel JC (2018) Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 15(3):541–553. https://doi.org/10.1007/s13311-018-0631-6
Uezono N, Zhu Y, Fujimoto Y, Yasui T, Matsuda T, Nakajo M, Abematsu M, Setoguchi T et al (2018) Prior Treatment with Anti-High Mobility Group Box-1 Antibody Boosts Human Neural Stem Cell Transplantation-Mediated Functional Recovery After Spinal Cord Injury. Stem Cells 36(5):737–750. https://doi.org/10.1002/stem.2802
Urban N, Blomfield IM, Guillemot F (2019) Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 104(5):834–848. https://doi.org/10.1016/j.neuron.2019.09.026
Parr AM, Kulbatski I, Zahir T, Wang X, Yue C, Keating A, Tator CH (2008) Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience 155(3):760–770. https://doi.org/10.1016/j.neuroscience.2008.05.042
Zhu Y, Uezono N, Yasui T, Nakashima K (2018) Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev Dyn 247(1):75–84. https://doi.org/10.1002/dvdy.24558
Grade S, Gotz M (2017) Neuronal replacement therapy: previous achievements and challenges ahead. NPJ Regen Med 2:29. https://doi.org/10.1038/s41536-017-0033-0
Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, Lin W, Li Y et al (2018) Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-kappaB pathway dependent of HDAC3. J Neuroinflammation 15(1):150. https://doi.org/10.1186/s12974-018-1193-6
Lee E, Hwang I, Park S, Hong S, Hwang B, Cho Y, Son J, Yu JW (2019) MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ 26(2):213–228. https://doi.org/10.1038/s41418-018-0124-5
Acknowledgements
We would like to acknowledge Jing An, Xiaohui Wang, and Lingling Zhang for the excellent technical assistance. We would like to thank Dr. Huiming Xu for critical reading of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 82071551, 82060238, and 81830077), the Natural Science Foundation of Shaanxi province (grant no. 2020JM-686), the key research and development program in Ning xia Hui Autonomous Region (grant no. 2022BEG02032).
Author information
Authors and Affiliations
Contributions
HY and JL conceived the study and designed the work. YH, YJ, LD, and LZ performed the experiments. YJ, LD, and CJ collected and analyzed the data. YH, JL, and HY performed data analysis and interpretation. YH and HY wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal procedures were performed in agreement with the National Institutes of Health guidelines and were approved by the Institutional Ethical Review Committee of Hong Hui Hospital affiliated by Xi’an Jiaotong University (No. 201712004). Written informed consent was obtained from all participants involved in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Y., Jiang, Y., Dong, L. et al. The aOECs Facilitate the Neuronal Differentiation of Neural Stem Cells in the Inflammatory Microenvironment Through Up-Regulation of Bioactive Factors and Activation of Wnt3/β-Catenin Pathway. Mol Neurobiol 60, 789–806 (2023). https://doi.org/10.1007/s12035-022-03113-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03113-w