Abstract
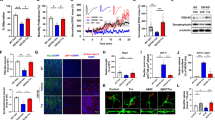
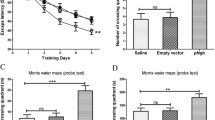
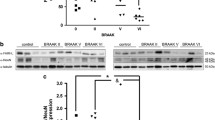
Neuronal apoptosis is considered to be a critical cause of Alzheimer’s disease (AD). Recently, meloxicam has shown neuroprotective effects; however, the inherent mechanisms are highly overlooked. Using APP/PS1 transgenic (Tg) mice as in vivo animal models, we found that meloxicam inhibits apoptosis in neurons by deactivating tumor necrosis factor receptor superfamily member 25 (TNFRSF25), leading to the suppression of the expression of fas-associated protein with death domain (FADD) and the cleavage of DNA fragmentation factor subunit α (DFFA) and cysteine aspartic acid protease-3 (caspase 3) via β-amyloid protein (Aβ)-depressing mechanisms. Moreover, the meloxicam treatment blocked the effects of β-amyloid protein oligomers (Aβo) on stimulating the synthesis of tumor necrosis factor α (TNF-α) and TNF-like ligand 1A (TL1A) in neuroblastoma (N) 2a cells. TNF-α and TL1A induce apoptosis in neurons via TNFR- and TNFRSF25-dependent caspase 3-activating mechanisms, respectively. Knocking down the expression of TNFRSF25 blocked the effects of TL1A on inducing apoptosis in neurons by deactivating the signaling cascades of FADD, caspase 3, and DFFA. Consistently, TNFRSF25 shRNA blocked the effects of Aβo on inducing neuronal apoptosis, which was corroborated by the efficacy of meloxicam in inhibiting Aβo-induced neuronal apoptosis. By ameliorating neuronal apoptosis, meloxicam improved memory loss in APP/PS1 Tg mice.








Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Andreasen N, Sjogren M, Blennow K (2015) CSF markers for Alzheimer’s disease: total tau, phospho-tau and Abeta42. World Journal of Biological Psychiatry 4(4):147–155
Duijn C, Clayton DG, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Mortimer JA (1994) Interaction between genetic and environmental risk factors for Alzheimer’s disease: a reanalysis of case-control studies. Genet Epidemiol 11(6):539–551
Knobloch M, Mansuy IM (2008) Dendritic spine loss and synaptic alterations in Alzheimer’s disease. Mol Neurobiol 37(1):73–82
Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, Niedzielko TL, Schneider LE, Mastroeni D, Caselli R (2008) Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci USA 105(11):4441–4446
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biol Med 23(1):134–147
Siegel GJ, Chauhan NB (2000) Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev 33(2–3):199–227
Kril JJ, Patel S, Harding AJ, Halliday GM (2002) Neuron loss from the hippocampus of Alzheimer’s disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol 103(4):370–376
Schmitz C, Rutten B, Pielen A, Schfer S, Bayer TA (2004) Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer’s disease. Am J Pathol 164(4):1495–1502
Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson PS, Hagberg H, Culmsee C (2007) Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ 14(4):775–784
Cosentino M, Colombo C, Mauri M, Fe Rrari M, Corbetta S, Marino F, Bono G, Lecchini S (2009) Expression of apoptosis-related proteins and of mRNA for dopaminergic receptors in peripheral blood mononuclear cells from patients with Alzheimer disease. Alzheimer Dis Assoc Disord 23(1):88–90
Guo XD, Sun GL, Zhou TT, Wang YY, Xu X, Shi XF, Zhu ZY, Rukachaisirikul V, Hu LH, Shen X (2017) LX2343 alleviates cognitive impairments in AD model rats by inhibiting oxidative stress-induced neuronal apoptosis and tauopathy. Acta Pharmacol Sin 38(008):1104–1119
Macgibbon GA, Lawlor PA, Sirimanne ES, Walton MR, Dragunow M (1997) Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer’s disease hippocampus. Brain Res 750(1–2):223–234
Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE (2010) Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol 8(1):65–72
Ashkenazi A, Salvesen G (2014) Regulated cell death: signaling and mechanisms. Annu Rev Cell Dev Biol 30(1):337–356
Steller H (1995) Mechanisms and genes of cellular suicide. Science 267(5203):1445–1449
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan KM (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123
He S, Lai W, Miao L, Tao W, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137(6):1100–1111
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325(5938):332–336
Migone TS, Zhang J, Luo X, Zhuang L, Wei P (2002) TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 16(3):479–492
Meylan F, Hayes E, T, Gabay, Odile, Richard, Arianne, C, (2015) The TNF-family cytokine TL1A: from lymphocyte costimulator to disease co-conspirator. J Leukoc Biol An Official Publ Reticuloendothel Soc 98(3):333–345
Bittner S, Knoll G, Fullsack S, Kurz M, Wajant H, Ehrenschwender M (2016) Soluble TL1A is sufficient for activation of death receptor 3. FEBS J 283(2):323–336
Chinnaiyan AM, O’Rourke K, Yu GL, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM (1996) Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 274(5289):990–992
Leng W, Zhuang L, Luo X, Ping W (2003) TL1A-induced NF-κB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem 278(40):39251–39258
Collins FL, Williams JO, Bloom AC, Stone MD, Choy E, Wang ECY, Williams AS (2015) Death receptor 3 (TNFRSF25) increases mineral apposition by osteoblasts and region specific new bone formation in the axial skeleton of male DBA/1 mice. Journal of Immunology Research 2015 (Special):901679
Dechant MJ, Fellenberg J, Scheuerpflug CG, Ewerbeck V, Debatin KM (2004) Mutation analysis of the apoptotic “death-receptors” and the adaptors TRADD and FADD/MORT-1 in osteosarcoma tumor samples and osteosarcoma cell lines. Int J Cancer 109(5):661–667
Chaudhary PM (1997) Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7(6):821–830
Haile M, Boutajangout A, Chung K, Chan J, Wisniewski T (2016) The Cox-2 inhibitor meloxicam ameliorates neuroinflammation and depressive behavior in adult mice after splenectomy. Journal of Neurophysiology & Neurological Disorders 3:101
Li J, Chen X, Dong X, Xu Z, Sun X (2010) Specific COX-2 inhibitor, meloxicam, suppresses proliferation and induces apoptosis in human HepG2 hepatocellular carcinoma cells. J Gastroenterol Hepatol 21(12):1814–1820
Goverdhan P, Sravanthi A, Mamatha T (2014) Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. International Journal of Alzheimers Disease 2012:974013
Ianiski FR, Alves CB, Ferreira CF, Rech VC, Savegnago L, Wilhelm EA, Luchese C (2016) Meloxicam-loaded nanocapsules as an alternative to improve memory decline in an Alzheimer’s disease model in mice: involvement of Na(+), K(+)-ATPase. Metab Brain Dis 31(4):793–802
Teismann P, Ferger B (2001) Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse 39(2):167–174
Katary MM, Pye C, Elmarakby AA (2016) Meloxicam fails to augment the reno-protective effects of soluble epoxide hydrolase inhibition in streptozotocin-induced diabetic rats via increased 20-HETE levels. Prostaglandins Other Lipid Mediat 132:3–11
Fortin JS, Benoit-Biancamano MO (2016) Inhibition of islet amyloid polypeptide aggregation and associated cytotoxicity by nonsteroidal anti-inflammatory drugs. Can J Physiol Pharmacol 94(1):35–48
Park JH, Park YS, Lee J, Park K, Paik M, Jeong M, Koh HC (2016) Meloxicam inhibits fipronil-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. J Appl Toxicol 36(1):10–23
Edfawy M, Hassan MH, Mansour A, Hamed AA, Amin H (2012) Meloxicam modulates oxidative stress status, inhibits prostaglandin E2, and abrogates apoptosis in carbon tetrachloride-induced rat hepatic injury. Int J Toxicol 31(3):276–286
Casas C, Sergeant N, Itier JM, Blanchard V, Pradier L (2004) Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Aβ42 accumulation in a novel Alzheimer transgenic model. Am J Pathol 165(4):1289–1300
Uetsuki T, Takemoto K, Nishimura I, Okamoto M, Niinobe M, Momoi T, Miura M, Yoshikawa K (1999) Activation of neuronal caspase-3 by intracellular accumulation of wild-type Alzheimer amyloid precursor protein. J Neurosci 19(16):6955–6964
Li W, Li B, Giacalone NJ, Torossian A, Sun Y, Niu K, Lin-Tsai O, Lu B (2011) BV6, an IAP antagonist, activates apoptosis and enhances radiosensitization of non-small cell lung carcinoma in vitro. Journal of Thoracic Oncology Official Publication of the International Association for the Study of Lung Cancer 6(11):1801–1809
Listed N (1999) Dementia and Alzheimer’s disease. Postgrad Med 106(5):161–162
Fang J, Wang L, Wu T, Yang C, Gao L, Cai H, Liu J, Fang S, Chen Y, Tan W (2016) Network pharmacology-based study on the mechanism of action for herbal medicines in Alzheimer treatment. J Ethnopharmacol 196:281–292
Tramutola A, Domenico FD, Barone E, Giorgi A, Francesco LD, Schinina E, Coccia R, Arena A, Head E, Butterfield DA (2017) Polyubiquitinylation profile in down syndrome brain before and after the development of Alzheimer neuropathology. Antioxid Redox Signal 26(7):280–298
Verhulsdonk S, Lange-Asschenfeldt C, Hoft B, Schwender H, Kalbe E (2017) Repressive coping does not contribute to anosognosia in first-diagnosis patients with Alzheimer disease. Alzheimer Dis Assoc Disord 31(3):249–255
Yong S, Ping H, Zhong Z, McAllister C, Lindholm K (2006) Distinct destructive signal pathways of neuronal death in Alzheimer’s disease. Trends Mol Med 12(12):574–579
Degterev A, Linkermann A (2016) Generation of small molecules to interfere with regulated necrosis. Cellular & Molecular Life Sciences Cmls 73(11–12):1–17
Li H, Ying L, Ying X, Yang L, Hu C, Chen Q, Yang Y, Jie M, Zhang J, Hui X (2018) Meloxicam improves cognitive impairment of diabetic rats through COX2-PGE2-EPs-cAMP/pPKA pathway. Mol Pharm 15(9):4121–4131
Yu L, Jiang R, Su Q, Yu H, Yang J (2014) Hippocampal neuronal metal ion imbalance related oxidative stress in a rat model of chronic aluminum exposure and neuroprotection of meloxicam. Behav Brain Funct 10(1):6
Tasaki Y, Omura T, Yamada T, Ohkubo T, Suno M, Iida S, Sakaguchi T, Asari M, Shimizu K, Matsubara K (2010) Meloxicam protects cell damage from 1-methyl-4-phenyl pyridinium toxicity via the phosphatidylinositol 3-kinase/Akt pathway in human dopaminergic neuroblastoma SH-SY5Y cells. Brain Res 1344(1):25–33
Novakova I, Subileau EA, Toegel S, Gruber D, Lachmann B, Urban E, Chesne C, Noe CR, Neuhaus W (2014) Transport rankings of non-steroidal antiinflammatory drugs across blood-brain barrier in vitro models. PLoS ONE 9(1):e86806. https://doi.org/10.1371/journal.pone.0086806
Maslanka T, Jaroszewski JJ, Markiewicz W, Jakubowski P (2010) Evaluation of the influence of meloxicam and flunixin meglumine on the apoptosis of peripheral blood CD4+ and CD8+ T cells in calves. Pol J Vet Sci 13(1):3–12
Cheng X, Zhao L, Ke T, Wang X, Cao L, Liu S, He J, Rong W (2021) Celecoxib ameliorates diabetic neuropathy by decreasing apoptosis and oxidative stress in dorsal root ganglion neurons via the miR-155/COX-2 axis. Exp Ther Med 22(2):825. https://doi.org/10.3892/etm.2021.10257
Dehlaghi Jadid K, Davidsson J, Lidin E, Hånell A, Angéria M, Mathiesen T, Risling M, Günther M (2019) COX-2 Inhibition by diclofenac is associated with decreased apoptosis and lesion area after experimental focal penetrating traumatic brain injury in rats. Front Neurol 10:811. https://doi.org/10.3389/fneur.2019.00811
Mazumder MK, Borah A (2014) Piroxicam inhibits NMDA receptor-mediated excitotoxicity through allosteric inhibition of the GluN2B subunit: an in silico study elucidating a novel mechanism of action of the drug. Med Hypotheses 83(6):740–746. https://doi.org/10.1016/j.mehy.2014.09.031
Holler N, Zaru R, Micheau O, Thome M, Tschopp J (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1(6):489–495
Laster SM, Wood JG, Gooding LR (1988) Tumor necrosis factor can induce both apoptic and necrotic forms of cell-lysis. J Immunol 141(8):2629–2634
Burne MJ, Elghandour A, Haq M, Saba SR, Norman J, Condon T, Bennett F, Rabb H (2001) IL-1 and TNF independent pathways mediate ICAM-1/VCAM-1 up-regulation in ischemia reperfusion injury. J Leukoc Biol 70(2):192–198
Perry RT, Collins JS, Wiener H, Acton R, Go RC (2001) The role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging 22(6):873–883. https://doi.org/10.1016/s0197-4580(01)00291-3
Samidurai M, Ramasamy VS, Jo J (2018) β-amyloid inhibits hippocampal LTP through TNFR/IKK/NF-κB pathway. Neurol Res 40(4):268–276. https://doi.org/10.1080/01616412.2018.1436872
Zhao A, Li Y, Deng Y (2020) TNF receptors are associated with tau pathology and conversion to Alzheimer’s dementia in subjects with mild cognitive impairment. Neurosci Lett 738:135392. https://doi.org/10.1016/j.neulet.2020.135392
Edgünlü TG, Ozge A, Yalın O, Kul S, Erdal ME (2013) A study of the impact of death receptor 4 (DR4) gene polymorphisms in Alzheimer’s disease. Balkan Med J 30(3):268–272. https://doi.org/10.5152/balkanmedj.2013.7455
Fossati S, Ghiso J, Rostagno A (2012) TRAIL death receptors DR4 and DR5 mediate cerebral microvascular endothelial cell apoptosis induced by oligomeric Alzheimer’s Aβ. Cell Death Dis 3(6):e321. https://doi.org/10.1038/cddis.2012.55
Dik B, Coskun D, Bahcivan E, Er A (2019) Doxycycline and meloxicam can treat neuroinflammation by increasing activity of antioxidant enzymes in rat brain. Pak J Pharm Sci 32(1):391–396
Kamer AR, Galoyan SM, Haile M, Kline R, Boutajangout A, Li YS, Bekker A (2012) Meloxicam improves object recognition memory and modulates glial activation after splenectomy in mice. Europ J Anaesthesiol EJA 29(7):332–337
Mehlhorn G, Hollborn M, Schliebs R (2000) Induction of cytokines in glial cells surrounding cortical β-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci 18(4–5):423–431
Khotib J, Utami NW, Gani MA, Ardianto C (2019) The change of proinflammatory cytokine tumor necrosis factor 伪 level in the use of meloxicam in rat model of osteoarthritis. J Basic Clin Physiol Pharmacol 30(6):185–193
Taha NF, Mahmoud KM, Soliman A, Emara LH (2021) Anti-inflammatory and cytoprotective potentials of meloxicam solid dispersions prepared by different techniques on lipopolysaccharide-stimulated RAW 264.7 macrophages. Journal of Drug Delivery Science and Technology 63(3):102507
Huang Y, Xu W, Zhou R (2021) NLRP3 inflammasome activation and cell death. Cell Mol Immunol 18(9):2114–2127. https://doi.org/10.1038/s41423-021-00740-6
Muhammad T, Ikram M, Ullah R, Rehman SU, Kim MO (2019) Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients 11(3):648. https://doi.org/10.3390/nu11030648
Zhang YY, Dong LX, Bao HL, Liu Y, An FM, Zhang GW (2021) Inhibition of interleukin-1β plays a protective role in Alzheimer’s disease by promoting microRNA-9-5p and downregulating targeting protein for xenopus kinesin-like protein 2. Int Immunopharmacol 97:107578. https://doi.org/10.1016/j.intimp.2021.107578
Suryawanshi A, Rouse BT, Niki T, Hirashima M, Podack ER (2012) TNFRSF25 Agonistic antibody and galectin-9 combination therapy controls herpes simplex virus-induced immunoinflammatory lesions. J Virol 86(19):10606–10620
Al-Lamki RS, Wang J, Tolkovsky AM, Bradley JA, Griffin JL, Thiru S, Wang EC, Bolton E, Min W, Moore P, Pober JS, Bradley JR (2008) TL1A both promotes and protects from renal inflammation and injury. J Am Soc Nephrol 19(5):953–960. https://doi.org/10.1681/ASN.2007060706 (ASN.2007060706 [pii])
Nilsson S (2004) Mutations in the N-terminal domain of DFF45 in a primary germ cell tumor and in neuroblastoma tumors. Int J Oncol 25(5):1297–1302
McCarty JS, Shen YT, Peng L (1999) Multiple domains of DFF45 bind synergistically to DFF40: roles of caspase cleavage and sequestration of activator domain of DFF40. Biochem Biophys Res Commun 264(1):181–185
Brustmann H (2006) DNA fragmentation factor (DFF45): Expression and prognostic value in serous ovarian cancer. Pathology - Research and Practice 202(10):713–720
Mukae N, Enari M, Sakahira H, Fukuda Y, Inazawa J, Toh H, Nagata S (1998) Molecular cloning and characterization of human caspase-activated DNase. Proc Natl Acad Sci USA 95(16):9123–9128
McCarty JS, Shen YT, Peng L (1999) Study of DFF45 in its role of chaperone and inhibitor: two independent inhibitory domains of DFF40 nuclease activity. Biochem Biophys Res Commun 264(1):176–180
Zhou P, Lugovskoy AA, McCarty JS, Li P, Wagner G (2001) Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45. Proc Natl Acad Sci USA 98(11):6051–6055. https://doi.org/10.1073/pnas.111145098
Eui-Jeon, Woo, and, Yeon-Gil, Kim, and, Min-Sung, Kim, and, Won-Deok (2004) Structural mechanism for inactivation and activation of CAD/DFF40 in the apoptotic pathway. Mol Cell 14(4):31–539
Behl C (2000) Apoptosis and Alzheimer’s disease. J Neural Transm 107(11):1325–1344. https://doi.org/10.1007/s007020070021
Funding
This work was supported, in part or in whole, by the National Natural Science Foundation of China (CN) (81870840).
Author information
Authors and Affiliations
Contributions
P.P.G. and D.Z. conceived and performed all of the experiments and participated in the design of the study. P.W. interpreted the data and wrote the manuscript of this study.
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted in accordance with the guidelines of the laboratory animal ethical standards and approved by the Ethics Committee of Northeastern University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guan, P., Zhu, D. & Wang, P. Meloxicam Inhibits Apoptosis in Neurons by Deactivating Tumor Necrosis Factor Receptor Superfamily Member 25, Leading to the Decreased Cleavage of DNA Fragmentation Factor Subunit α in Alzheimer’s Disease. Mol Neurobiol 60, 395–412 (2023). https://doi.org/10.1007/s12035-022-03091-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03091-z