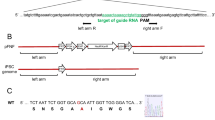
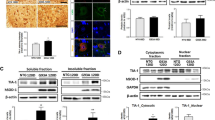
Abstract
Although a couple of studies have reported that mutant superoxide dismutase 1 (SOD1), one of the causative genes of familial amyotrophic lateral, interacts physically with lysyl-tRNA synthetase (KARS1) by a gain of function, there is limited evidence regarding the detailed mechanism about how the interaction leads to neuronal cell death. Our results indicated that the aminoacyl-tRNA synthetase-interacting multi-functional protein 2 (AIMP2) mediated cell death upon the interplay between mutant SOD1 and KARS1 in ALS. Binding of mutant SOD1 with KARS1 led to the release of AIMP2 from its original binding partner KARS1, and the free form of AIMP2 induced TRAF2 degradation followed by TNF-α-induced cell death. We also suggest a therapeutic application that overexpression of DX2, the exon 2-deleted antagonistic splicing variant of AIMP2 (AIMP2-DX2), reduced neuronal cell death in the ALS mouse model. Expression of DX2 suppressed TRAF2 degradation and TNF-α-induced cell death by competing mode of action against full-length AIMP2. Motor neuron differentiated form iPSC showed a resistance in neuronal cell death after DX2 administration. Further, intrathecal administration of DX2-coding adeno-associated virus (AAV) improved locomotive activity and survival in a mutant SOD1-induced ALS mouse model. Taken together, these results indicated that DX2 could prolong life span and delay the ALS symptoms through compensation in neuronal inflammation.







Similar content being viewed by others
Data Availability
Most of datasets generated or analyzed during the current study are included in this published article and available from the corresponding author on reasonable request.
Abbreviations
- AAV:
-
Adeno-associated virus
- AIMP2:
-
Aminoacyl-tRNA synthetase-interacting multi-functional protein 2
- ALS:
-
Amyotrophic lateral sclerosis
- DX2:
-
Exon 2-deleted splicing variant of AIMP2
- KARS1:
-
Lysyl-tRNA synthetase
- MSC:
-
multi-tRNA synthetase complex
- SOD1:
-
superoxide dismutase 1
- TNF-α:
-
tumor necrosis factor-α
- TRAF2:
-
TNF receptor-associated factor 2
References
Al-Chalabi A, Hardiman O (2013) The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 9(11):617–628. https://doi.org/10.1038/nrneurol.2013.203
van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, van den Berg LH (2017) Amyotrophic lateral sclerosis. Lancet 390(10107):2084–2098. https://doi.org/10.1016/S0140-6736(17)31287-4
Murdock BJ, Bender DE, Segal BM, Feldman EL (2015) The dual roles of immunity in ALS: injury overrides protection. Neurobiol Dis 77:1–12. https://doi.org/10.1016/j.nbd.2015.02.017
Pasinelli P, Brown RH (2006) Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7(9):710–723. https://doi.org/10.1038/nrn1971
Bucchia M, Ramirez A, Parente V, Simone C, Nizzardo M, Magri F, Dametti S, Corti S (2015) Therapeutic development in amyotrophic lateral sclerosis. Clin Ther 37(3):668–680. https://doi.org/10.1016/j.clinthera.2014.12.020
Miller RG, Mitchell JD, Moore DH (2001) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). The Cochrane database of systematic reviews (4):CD001447. https://doi.org/10.1002/14651858.CD001447
Rothstein JD (2017) Edaravone: a new drug approved for ALS. Cell 171(4):725–726. https://doi.org/10.1016/j.cell.2017.10.011
Lunn JS, Sakowski SA, Feldman EL (2014) Concise review: stem cell therapies for amyotrophic lateral sclerosis: recent advances and prospects for the future. Stem cells 32(5):1099–1109. https://doi.org/10.1002/stem.1628
Writing G, Edaravone ALSSG (2017) Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 16(7):505–512. https://doi.org/10.1016/S1474-4422(17)30115-1
Martinez A, Palomo Ruiz MD, Perez DI, Gil C (2017) Drugs in clinical development for the treatment of amyotrophic lateral sclerosis. Expert Opin Investig Drugs 26(4):403–414. https://doi.org/10.1080/13543784.2017.1302426
Gagliardi S, Cova E, Davin A, Guareschi S, Abel K, Alvisi E, Laforenza U, Ghidoni R, et al (2010) SOD1 mRNA expression in sporadic amyotrophic lateral sclerosis. Neurobiol Dis 39(2):198–203. https://doi.org/10.1016/j.nbd.2010.04.008
Roggenbuck J, Quick A, Kolb SJ (2017) Genetic testing and genetic counseling for amyotrophic lateral sclerosis: an update for clinicians. Genet Med 19(3):267–274. https://doi.org/10.1038/gim.2016.107
Allen MJ, Lacroix JJ, Ramachandran S, Capone R, Whitlock JL, Ghadge GD, Arnsdorf MF, Roos RP, et al (2012) Mutant SOD1 forms ion channel: implications for ALS pathophysiology. Neurobiol Dis 45(3):831–838. https://doi.org/10.1016/j.nbd.2011.08.031
Hensley K, Abdel-Moaty H, Hunter J, Mhatre M, Mou S, Nguyen K, Potapova T, Pye QN, et al (2006) Primary glia expressing the G93A-SOD1 mutation present a neuroinflammatory phenotype and provide a cellular system for studies of glial inflammation. J Neuroinflammation 3:2. https://doi.org/10.1186/1742-2094-3-2
Gowing G, Dequen F, Soucy G, Julien JP (2006) Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J Neurosci 26(44):11397–11402. https://doi.org/10.1523/JNEUROSCI.0602-06.2006
Petri S, Kiaei M, Wille E, Calingasan NY, Flint Beal M (2006) Loss of Fas ligand-function improves survival in G93A-transgenic ALS mice. J Neurol Sci 251(1–2):44–49. https://doi.org/10.1016/j.jns.2006.08.013
Hensley K, Fedynyshyn J, Ferrell S, Floyd RA, Gordon B, Grammas P, Hamdheydari L, Mhatre M, et al (2003) Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis 14(1):74–80. https://doi.org/10.1016/s0969-9961(03)00087-1
Pare B, Lehmann M, Beaudin M, Nordstrom U, Saikali S, Julien JP, Gilthorpe JD, Marklund SL, et al (2018) Misfolded SOD1 pathology in sporadic amyotrophic lateral sclerosis. Sci Rep 8(1):14223. https://doi.org/10.1038/s41598-018-31773-z
Munch C, O’Brien J, Bertolotti A (2011) Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci USA 108(9):3548–3553. https://doi.org/10.1073/pnas.1017275108
Boillee S, Vande Velde C, Cleveland DW (2006) ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52(1):39–59. https://doi.org/10.1016/j.neuron.2006.09.018
Kaur SJ, McKeown SR, Rashid S (2016) Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene 577(2):109–118. https://doi.org/10.1016/j.gene.2015.11.049
Saccon RA, Bunton-Stasyshyn RK, Fisher EM, Fratta P (2013) Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain 136(Pt 8):2342–2358. https://doi.org/10.1093/brain/awt097
Kunst CB, Mezey E, Brownstein MJ, Patterson D (1997) Mutations in SOD1 associated with amyotrophic lateral sclerosis cause novel protein interactions. Nat Genet 15(1):91–94. https://doi.org/10.1038/ng0197-91
Lee SW, Cho BH, Park SG, Kim S (2004) Aminoacyl-tRNA synthetase complexes: beyond translation. J Cell Sci 117(Pt 17):3725–3734. https://doi.org/10.1242/jcs.01342
Park SG, Ewalt KL, Kim S (2005) Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci 30(10):569–574. https://doi.org/10.1016/j.tibs.2005.08.004
Kim DG, Choi JW, Lee JY, Kim H, Oh YS, Lee JW, Tak YK, Song JM, et al (2012) Interaction of two translational components, lysyl-tRNA synthetase and p40/37LRP, in plasma membrane promotes laminin-dependent cell migration. FASEB J 26(10):4142–4159. https://doi.org/10.1096/fj.12-207639
Kim DG, Lee JY, Kwon NH, Fang P, Zhang Q, Wang J, Young NL, Guo M, et al (2014) Chemical inhibition of prometastatic lysyl-tRNA synthetase-laminin receptor interaction. Nat Chem Biol 10(1):29–34. https://doi.org/10.1038/nchembio.1381
Lee YN, Nechushtan H, Figov N, Razin E (2004) The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcepsilonRI-activated mast cells. Immunity 20(2):145–151. https://doi.org/10.1016/s1074-7613(04)00020-2
Hei Z, Wu S, Liu Z, Wang J, Fang P (2019) Retractile lysyl-tRNA synthetase-AIMP2 assembly in the human multi-aminoacyl-tRNA synthetase complex. J Biol Chem 294(13):4775–4783. https://doi.org/10.1074/jbc.RA118.006356
Han JM, Park BJ, Park SG, Oh YS, Choi SJ, Lee SW, Hwang SK, Chang SH, et al (2008) AIMP2/p38, the scaffold for the multi-tRNA synthetase complex, responds to genotoxic stresses via p53. Proc Natl Acad Sci USA 105(32):11206–11211. https://doi.org/10.1073/pnas.0800297105
Lee HS, Kim DG, Oh YS, Kwon NH, Lee JY, Kim D, Park SH, Song JH, et al (2013) Chemical suppression of an oncogenic splicing variant of AIMP2 induces tumour regression. Biochem J 454(3):411–416. https://doi.org/10.1042/BJ20130550
Choi JW, Kim DG, Park MC, Um JY, Han JM, Park SG, Choi EC, Kim S (2009) AIMP2 promotes TNFalpha-dependent apoptosis via ubiquitin-mediated degradation of TRAF2. J Cell Sci 122(Pt 15):2710–2715. https://doi.org/10.1242/jcs.049767
Choi JW, Um JY, Kundu JK, Surh YJ, Kim S (2009) Multidirectional tumor-suppressive activity of AIMP2/p38 and the enhanced susceptibility of AIMP2 heterozygous mice to carcinogenesis. Carcinogenesis 30(9):1638–1644. https://doi.org/10.1093/carcin/bgp170
Choi JW, Kim DG, Lee AE, Kim HR, Lee JY, Kwon NH, Shin YK, Hwang SK, et al (2011) Cancer-associated splicing variant of tumor suppressor AIMP2/p38: pathological implication in tumorigenesis. PLoS Genet 7(3):e1001351. https://doi.org/10.1371/journal.pgen.1001351
Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, Henderson CE, Haase G (2006) Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc Natl Acad Sci USA 103(15):6007–6012. https://doi.org/10.1073/pnas.0508774103
Beier CP, Wischhusen J, Gleichmann M, Gerhardt E, Pekanovic A, Krueger A, Taylor V, Suter U, et al (2005) FasL (CD95L/APO-1L) resistance of neurons mediated by phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent expression of lifeguard/neuronal membrane protein 35. J Neurosci 25(29):6765–6774. https://doi.org/10.1523/JNEUROSCI.1700-05.2005
Aebischer J, Bernard-Marissal N, Pettmann B, Raoul C (2013) Death receptors in the selective degeneration of motoneurons in amyotrophic lateral sclerosis. J Neurodegener Dis 2013:746845. https://doi.org/10.1155/2013/746845
Tortarolo M, Lo Coco D, Veglianese P, Vallarola A, Giordana MT, Marcon G, Beghi E, Poloni M, et al (2017) Amyotrophic lateral sclerosis, a multisystem pathology: insights into the role of TNFalpha. Mediators Inflamm 2017:2985051. https://doi.org/10.1155/2017/2985051
Guidotti G, Scarlata C, Brambilla L, Rossi D (2021) Tumor necrosis factor alpha in amyotrophic lateral sclerosis: friend or foe? Cells 10(3). https://doi.org/10.3390/cells10030518
Brohawn DG, O’Brien LC, Bennett JP Jr (2016) RNAseq analyses identify tumor necrosis factor-mediated inflammation as a major abnormality in ALS spinal cord. PLoS ONE 11(8):e0160520. https://doi.org/10.1371/journal.pone.0160520
Azzouz M, Hottinger A, Paterna JC, Zurn AD, Aebischer P, Bueler H (2000) Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum Mol Genet 9(5):803–811. https://doi.org/10.1093/hmg/9.5.803
Franz CK, Federici T, Yang J, Backus C, Oh SS, Teng Q, Carlton E, Bishop KM, et al (2009) Intraspinal cord delivery of IGF-I mediated by adeno-associated virus 2 is neuroprotective in a rat model of familial ALS. Neurobiol Dis 33(3):473–481. https://doi.org/10.1016/j.nbd.2008.12.003
Perez BA, Shutterly A, Chan YK, Byrne BJ, Corti M (2020) Management of neuroinflammatory responses to AAV-mediated gene therapies for neurodegenerative diseases. Brain Sci 10(2). https://doi.org/10.3390/brainsci10020119
Ratushny V, Golemis E (2008) Resolving the network of cell signaling pathways using the evolving yeast two-hybrid system. Biotechniques 44(5):655–662. https://doi.org/10.2144/000112797
Amoroso MW, Croft GF, Williams DJ, O’Keeffe S, Carrasco MA, Davis AR, Roybon L, Oakley DH, et al (2013) Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J Neurosci 33(2):574–586. https://doi.org/10.1523/JNEUROSCI.0906-12.2013
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357–359. https://doi.org/10.1038/nmeth.1923
Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26(6):841–842. https://doi.org/10.1093/bioinformatics/btq033
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, et al (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5(10):R80. https://doi.org/10.1186/gb-2004-5-10-r80
Lee JD, Kamaruzaman NA, Fung JN, Taylor SM, Turner BJ, Atkin JD, Woodruff TM, Noakes PG (2013) Dysregulation of the complement cascade in the hSOD1G93A transgenic mouse model of amyotrophic lateral sclerosis. J Neuroinflammation 10:119. https://doi.org/10.1186/1742-2094-10-119
Liu J, Wang F (2017) Role of neuroinflammation in amyotrophic lateral sclerosis: cellular mechanisms and therapeutic implications. Front Immunol 8:1005. https://doi.org/10.3389/fimmu.2017.01005
Geloso MC, Corvino V, Marchese E, Serrano A, Michetti F, D’Ambrosi N (2017) The dual role of microglia in ALS: mechanisms and therapeutic approaches. Front Aging Neurosci 9:242. https://doi.org/10.3389/fnagi.2017.00242
Choi JW, Lee JW, Kim JK, Jeon HK, Choi JJ, Kim DG, Kim BG, Nam DH, et al (2012) Splicing variant of AIMP2 as an effective target against chemoresistant ovarian cancer. J Mol Cell Biol 4(3):164–173. https://doi.org/10.1093/jmcb/mjs018
Linnerbauer M, Wheeler MA, Quintana FJ (2020) Astrocyte crosstalk in CNS inflammation. Neuron 108(4):608–622. https://doi.org/10.1016/j.neuron.2020.08.012
Ban J, Samano C, Mladinic M, Munitic I (2019) Glia in amyotrophic lateral sclerosis and spinal cord injury: common therapeutic targets. Croat Med J 60(2):109–120. https://doi.org/10.3325/cmj.2019.60.109
Voet S, Prinz M, van Loo G (2019) Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med 25(2):112–123. https://doi.org/10.1016/j.molmed.2018.11.005
Giovannoni F, Quintana FJ (2020) The role of astrocytes in CNS inflammation. Trends Immunol 41(9):805–819. https://doi.org/10.1016/j.it.2020.07.007
Kawamata H, Magrane J, Kunst C, King MP, Manfredi G (2008) Lysyl-tRNA synthetase is a target for mutant SOD1 toxicity in mitochondria. J Biol Chem 283(42):28321–28328. https://doi.org/10.1074/jbc.M805599200
Lee Y, Karuppagounder SS, Shin JH, Lee YI, Ko HS, Swing D, Jiang H, Kang SU, et al (2013) Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci 16(10):1392–1400. https://doi.org/10.1038/nn.3500
Li X, Yang Y, Ashwell JD (2002) TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 416(6878):345–347. https://doi.org/10.1038/416345a
Li J, Zhang J, Zhang Y, Wang Z, Song Y, Wei S, He M, You S, et al (2019) TRAF2 protects against cerebral ischemia-induced brain injury by suppressing necroptosis. Cell Death Dis 10(5):328. https://doi.org/10.1038/s41419-019-1558-5
Cereda C, Baiocchi C, Bongioanni P, Cova E, Guareschi S, Metelli MR, Rossi B, Sbalsi I, et al (2008) TNF and sTNFR1/2 plasma levels in ALS patients. J Neuroimmunol 194(1–2):123–131. https://doi.org/10.1016/j.jneuroim.2007.10.028
Kiaei M, Petri S, Kipiani K, Gardian G, Choi DK, Chen J, Calingasan NY, Schafer P, et al (2006) Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 26(9):2467–2473. https://doi.org/10.1523/JNEUROSCI.5253-05.2006
Barbaric I, Miller G, Dear TN (2007) Appearances can be deceiving: phenotypes of knockout mice. Brief Funct Genomic Proteomic 6(2):91–103. https://doi.org/10.1093/bfgp/elm008
Toder V, Fein A, Carp H, Torchinsky A (2003) TNF-alpha in pregnancy loss and embryo maldevelopment: a mediator of detrimental stimuli or a protector of the fetoplacental unit? J Assist Reprod Genet 20(2):73–81. https://doi.org/10.1023/a:1021740108284
Green DR, Llambi F (2015) Cell death signaling. Cold Spring Harb Perspect Biol 7(12). https://doi.org/10.1101/cshperspect.a006080
Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F, Hajjar RJ (2007) Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther 14(13):989–997. https://doi.org/10.1038/sj.gt.3302895
Funding
This work was supported by the Basic Science Research Program, Ministry of Science and ICT (NRF-2017R1A5A2014768, 2018R1A2B3008483, 2019R1A2C1006752, 2021R1A3B1076605, and NRF-2022R1A2C2009281); a grant from the Global Frontier Project (NRF-2016M3A6A4929906) of the National Research Foundation, Ministry of Science and ICT of Korea; and partially the Yonsei University Research Fund of 2020–22-0358, 2020–22-0356, and 2021–22-0061. And the research was supported by a grant (21153MFDS601) from Ministry of Food and Drug Safety in 2022.
Author information
Authors and Affiliations
Contributions
M. G. K., M. R. B., S. M. L., and J. W. C. analyzed the results and wrote the manuscript. M. R. B., M. G. K., S. M. L., H. B. L., M. H. L., and D. H. L. performed the experiments. S. H. K. and K. S. K. contributed to the interpretation and discussion of the results. K. H. B. and J. W. C. leaded the research project.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All experimental procedures were performed in accordance with guidelines of the Seoul National University Institutional Animal Care and Use Committee (SNUIACUC, August 7, 2017). Our local ethics committee “SNUIACUC” approved the present study (Approval No. SNU-170807–1).
Consent for Publication
Authors give consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kook, M.G., Byun, M.R., Lee, S.M. et al. Anti-apoptotic Splicing Variant of AIMP2 Recover Mutant SOD1-Induced Neuronal Cell Death. Mol Neurobiol 60, 145–159 (2023). https://doi.org/10.1007/s12035-022-03073-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03073-1