Abstract
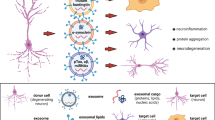
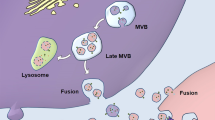
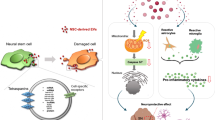
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease and a leading cause of dementia. Although the amyloid-β (Aβ) peptide is deemed a crucial driver of AD, there are no effective therapeutics available to treat Aβ-caused neurotoxicity. Extracellular vesicles (EVs) are membrane-bound small particles mediating intercellular traffic of nucleic acids, lipids, proteins, and metabolites. Exosomes are a subtype of EVs with a size range of 30–150 nm in diameter. Stem cell-derived EVs are a potential therapeutic for AD, while EVs isolated from normal stem cell cultures generally have a low yield. Here, we studied the EVs secreted by the rat neural stem cells in the presence of heat shock (HS) stimulus. Nanoparticle tracking analysis confirmed that HS-derived EVs exhibit significantly higher concentration and larger diameter in comparison to the non-heat shock (NHS)-derived EVs. Mass spectrometric studies of EV proteins revealed that HS-derived EVs contained fewer diverse proteins than NHS-derived exosomes. GO enrichment analysis of the proteins suggested that the top two biological functions of the proteins in HS-derived EVs are involved in the negative regulation of apoptotic process and positive modulation of DNA repair. Importantly, the therapeutic efficacy of the NHS- and HS-derived EVs were tested in a cell culture model of AD: HS-derived EVs exhibited greater neuroprotection against not only oxidative stress but also amyloid-β (Aβ) induced neurotoxicity compared to NHS-derived EVs. Moreover, HS-derived EVs were also able to dramatically attenuate Aβ-induced apoptosis and oxidative stress. These data indicate that in response to HS, neural stem cells increase EV production and alter EV morphology and cargo to confer better neuroprotection against oxidative stress and Aβ-caused neurotoxicity, suggesting that HS-induced EVs from neural stem cells can be a therapeutic agent for AD and possibly other neurological disorders.




Similar content being viewed by others
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
2021 Alzheimer’s disease facts and figures (2021). Alzheimers Dement 17 (3):327–406. https://doi.org/10.1002/alz.12328
Se Thoe E, Fauzi A, Tang YQ, Chamyuang S, Chia AYY (2021) A review on advances of treatment modalities for Alzheimer’s disease. Life Sci 276:119129. https://doi.org/10.1016/j.lfs.2021.119129
Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8(7):727. https://doi.org/10.3390/cells8070727
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J (2018) Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150:137–149. https://doi.org/10.1016/j.biomaterials.2017.10.012
Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y, Zheng Y (2021) Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 11(18):8926–8944. https://doi.org/10.7150/thno.62330
Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Gorgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Kramer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lotvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Thery C, Rohde E, Giebel B (2015) Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 4:30087. https://doi.org/10.3402/jev.v4.30087
Yamashita T, Takahashi Y, Takakura Y (2018) Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull 41(6):835–842. https://doi.org/10.1248/bpb.b18-00133
Hoshimaru M, Ray J, Sah DW, Gage FH (1996) Differentiation of the immortalized adult neuronal progenitor cell line HC2S2 into neurons by regulatable suppression of the v-myc oncogene. Proc Natl Acad Sci U S A 93(4):1518–1523. https://doi.org/10.1073/pnas.93.4.1518
Dong G, Ferguson JM, Duling AJ, Nicholas RG, Zhang D, Rezvani K, Fang S, Monteiro MJ, Li S, Li XJ, Wang H (2011) Modeling pathogenesis of Huntington’s disease with inducible neuroprogenitor cells. Cell Mol Neurobiol 31(5):737–747. https://doi.org/10.1007/s10571-011-9679-0
Hoshimaru M, Ray J, Sah DWY, Gage FH (1996) Differentiation of the immortalized adult neuronal progenitor cell line HC2S2 into neurons by regulatable suppression of the v-myc oncogene. P Natl Acad Sci USA 93(4):1518–1523. https://doi.org/10.1073/pnas.93.4.1518
Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z (2005) Induction of heat shock proteins in B-cell exosomes. J Cell Sci 118(Pt 16):3631–3638. https://doi.org/10.1242/jcs.02494
Liu Y, Huber CC, Wang H (2020) Disrupted blood-brain barrier in 5xFAD mouse model of Alzheimer’s disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2020.02.074
Liu Y, Subedi K, Baride A, Romanova S, Callegari E, Huber CC, Wang X, Wang H (2021) Peripherally misfolded proteins exacerbate ischemic stroke-induced neuroinflammation and brain injury. J Neuroinflammation 18(1):29. https://doi.org/10.1186/s12974-021-02081-7
van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, Sturk A, van Leeuwen TG, Nieuwland R (2014) Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost 12(7):1182–1192. https://doi.org/10.1111/jth.12602
Dong G, Callegari E, Gloeckner CJ, Ueffing M, Wang H (2012) Mass spectrometric identification of novel posttranslational modification sites in Huntingtin. Proteomics 12(12):2060–2064. https://doi.org/10.1002/pmic.201100380
Dong G, Callegari EA, Gloeckner CJ, Ueffing M, Wang H (2012) Prothymosin-alpha interacts with mutant huntingtin and suppresses its cytotoxicity in cell culture. J Biol Chem 287(2):1279–1289. https://doi.org/10.1074/jbc.M111.294280
Liu Y, Min JW, Feng S, Subedi K, Qiao F, Mammenga E, Callegari E, Wang H (2020) Therapeutic role of a cysteine precursor, OTC, in ischemic stroke is mediated by improved proteostasis in mice. Transl Stroke Res 11(1):147–160. https://doi.org/10.1007/s12975-019-00707-w
Liu Y, Qiao F, Wang H (2017) Enhanced proteostasis in post-ischemic stroke mouse brains by ubiquilin-1 promotes functional recovery. Cell Mol Neurobiol 37(7):1325–1329. https://doi.org/10.1007/s10571-016-0451-3
Stine WB, Jungbauer L, Yu C, LaDu MJ (2011) Preparing synthetic Abeta in different aggregation states. Methods Mol Biol 670:13–32. https://doi.org/10.1007/978-1-60761-744-0_2
Liu Y, Lu L, Hettinger CL, Dong G, Zhang D, Rezvani K, Wang X, Wang H (2014) Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. J Neurosci 34(8):2813–2821. https://doi.org/10.1523/JNEUROSCI.3541-13.2014
Chen L, Liu L, Yin J, Luo Y, Huang S (2009) Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol 41(6):1284–1295. https://doi.org/10.1016/j.biocel.2008.10.029
Yao M, Nguyen TV, Pike CJ (2005) Beta-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J Neurosci 25(5):1149–1158. https://doi.org/10.1523/JNEUROSCI.4736-04.2005
Liu Y, Qiao F, Wang H (2016) Enhanced proteostasis in post-ischemic stroke mouse brains by ubiquilin-1 promotes functional recovery. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-016-0451-3
Feng Y, Huang W, Meng W, Jegga AG, Wang Y, Cai W, Kim HW, Pasha Z, Wen Z, Rao F, Modi RM, Yu X, Ashraf M (2014) Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: a critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells 32(2):462–472. https://doi.org/10.1002/stem.1571
Peruzzotti-Jametti L, Bernstock JD, Willis CM, Manferrari G, Rogall R, Fernandez-Vizarra E, Williamson JC, Braga A, van den Bosch A, Leonardi T, Krzak G, Kittel A, Beninca C, Vicario N, Tan S, Bastos C, Bicci I, Iraci N, Smith JA, Peacock B, Muller KH, Lehner PJ, Buzas EI, Faria N, Zeviani M, Frezza C, Brisson A, Matheson NJ, Viscomi C, Pluchino S (2021) Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol 19(4):e3001166. https://doi.org/10.1371/journal.pbio.3001166
Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis 42(Suppl 3):S125-152. https://doi.org/10.3233/JAD-132738
Acknowledgements
We would like to thank Dr. Aravind Baride in the Department of Chemistry at the University of South Dakota for his assistance in electron microscopy.
Funding
This work was supported in part by the National Science Foundation (NSF) (DGE-1633213), NIH/NIGMS (T32GM-136503), NIH/NIGMS P20 GM103443-20, and NIH/NIA RF1 AG072510. Any opinions, findings, conclusions, or recommendations expressed in this material are those from the authors and do not necessarily reflect the views from the NSF or NIH.
Author information
Authors and Affiliations
Contributions
Christa C. Huber and Hongmin Wang contributed to the study conception and design. Experiments and data analysis were performed by Christa C. Huber, Eduardo A. Callegari, Maria D. Paez, Svetlana Romanova and Hongmin Wang. The first draft of the manuscript was written by Christa C. Huber. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huber, C.C., Callegari, E.A., Paez, M.D. et al. Heat Shock-Induced Extracellular Vesicles Derived from Neural Stem Cells Confer Marked Neuroprotection Against Oxidative Stress and Amyloid-β-Caused Neurotoxicity. Mol Neurobiol 59, 7404–7412 (2022). https://doi.org/10.1007/s12035-022-03055-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03055-3