Abstract
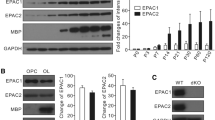
Nuclear inhibitor of protein phosphatase 1 (NIPP1) is a known regulator of gene expression and plays roles in many physiological or pathological processes such as stem cell proliferation and skin inflammation. While NIPP1 has many regulatory roles in proliferating cells, its function in the central nervous system (CNS) has not been directly investigated. In the present study, we examined NIPP1 CNS function using a conditional knockout (cKO) mouse model in which the Nipp1 gene is excised from neural precursor cells. These mice exhibited severe developmental impairments that led to premature lethality. To delineate the neurological changes occurring in these animals, we first assessed microtubule-associated protein tau, a known target of NIPP1 activity. We found that phosphorylation of tau is significantly enhanced in NIPP1 cKO mice. Consistent with this, we found altered AKT and PP1 activity in NIPP1 cKO mice, suggesting that increased tau phosphorylation likely results from a shift in kinase/phosphatase activity. Secondly, we observed tremors in the NIPP1 cKO mice which prompted us to explore the integrity of the myelin sheath, an integral structure for CNS function. We demonstrated that in NIPP1 cKO mice, there is a significant decrease in MBP protein expression in the cortex, along with deficits in both the conduction of compound action potentials (CAP) and the percentage of myelinated axons in the optic nerve. Our study suggests that NIPP1 in neural precursor cells regulates phosphorylation of tau and CNS myelination and may represent a novel therapeutic target for neurodegenerative diseases.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Trinkle-Mulcahy L, Ajuh P, Prescott A, Claverie-Martin F, Cohen S, Lamond AI, Cohen P (1999) Nuclear organisation of NIPP1, a regulatory subunit of protein phosphatase 1 that associates with pre-mRNA splicing factors. J Cell Sci 112(Pt 2):157–168
Winkler, C., R. Rouget, D. Wu, M. Beullens, A. Van Eynde, and M (2018) Bollen, Overexpression of PP1-NIPP1 limits the capacity of cells to repair DNA double-strand breaks. J Cell Sci 131(13).
Ferreira M, Verbinnen I, Fardilha M, Van Eynde A, Bollen M (2018) The deletion of the protein phosphatase 1 regulator NIPP1 in testis causes hyperphosphorylation and degradation of the histone methyltransferase EZH2. J Biol Chem 293(47):18031–18039
Jin Q, van Eynde A, Beullens M, Roy N, Thiel G, Stalmans W, Bollen M (2003) The protein phosphatase-1 (PP1) regulator, nuclear inhibitor of PP1 (NIPP1), interacts with the polycomb group protein, embryonic ectoderm development (EED), and functions as a transcriptional repressor. J Biol Chem 278(33):30677–30685
Minnebo N, Gornemann J, O’Connell N, Van Dessel N, Derua R, Vermunt MW, Page R et al (2013) NIPP1 maintains EZH2 phosphorylation and promoter occupancy at proliferation-related target genes. Nucleic Acids Res 41(2):842–854
Van Dessel N, Beke L, Gornemann J, Minnebo N, Beullens M, Tanuma N, Shima H, Van Eynde A, Bollen M (2010) The phosphatase interactor NIPP1 regulates the occupancy of the histone methyltransferase EZH2 at Polycomb targets. Nucleic Acids Res 38(21):7500–7512
Van Eynde A, Nuytten M, Dewerchin M, Schoonjans L, Keppens S, Beullens M, Moons L, Carmeliet P, Stalmans W, Bollen M (2004) The nuclear scaffold protein NIPP1 is essential for early embryonic development and cell proliferation. Mol Cell Biol 24(13):5863–5874
Boens S, Verbinnen I, Verhulst S, Szeker K, Ferreira M, Gevaert T, Baes M, Roskams T et al (2016) Brief report: the deletion of the phosphatase regulator NIPP1 causes progenitor cell expansion in the adult liver. Stem Cells 34(8):2256–2262
Ferreira M, Boens S, Winkler C, Szeker K, Verbinnen I, Van Eynde A, Fardilha M, Bollen M (2017) The protein phosphatase 1 regulator NIPP1 is essential for mammalian spermatogenesis. Sci Rep 7(1):13364
Verbinnen I, Jonkhout M, Liakath-Ali K, Szeker K, Ferreira M, Boens S, Rouget R, Nikolic M et al (2020) Phosphatase regulator NIPP1 restrains chemokine-driven skin inflammation. J Invest Dermatol 140(8):1576–1588
Novoyatleva T, Heinrich B, Tang Y, Benderska N, Butchbach ME, Lorson CL, Lorson MA, Ben-Dov C et al (2008) Protein phosphatase 1 binds to the RNA recognition motif of several splicing factors and regulates alternative pre-mRNA processing. Hum Mol Genet 17(1):52–70
Welden JR, van Doorn J, Nelson PT, Stamm S (2018) The human MAPT locus generates circular RNAs. Biochim Biophys Acta Mol Basis Dis 1864(9):2753–2760
Wang Y, Mandelkow E (2016) Tau in physiology and pathology. Nat Rev Neurosci 17(1):5–21
Braithwaite SP, Stock JB, Lombroso PJ, Nairn AC (2012) Protein phosphatases and Alzheimer’s disease. Prog Mol Biol Transl Sci 106:343–379
Readhead C, Hood L (1990) The dysmyelinating mouse mutations shiverer (shi) and myelin deficient (shimld). Behav Genet 20(2):213–234
Hou H, Sun L, Siddoway BA, Petralia RS, Yang H, Gu H, Nairn AC, Xia H (2013) Synaptic NMDA receptor stimulation activates PP1 by inhibiting its phosphorylation by Cdk5. J Cell Biol 203(3):521–535
Mayoral SR, Etxeberria A, Shen YA, Chan JR (2018) Initiation of CNS myelination in the optic nerve is dependent on axon caliber. Cell Rep 25(3):544–550 (e3)
Yang H, Hou H, Pahng A, Gu H, Nairn AC, Tang YP, Colombo PJ, Xia H (2015) Protein phosphatase-1 inhibitor-2 is a novel memory suppressor. J Neurosci 35(45):15082–15087
Dickson DW, Kouri N, Murray ME, Josephs KA (2011) Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 45(3):384–389
Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Mobius W, Goetze B et al (2016) Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91(1):119–132
McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD (2014) Motor skill learning requires active central myelination. Science 346(6207):318–322
Monje M (2018) Myelin plasticity and nervous system function. Annu Rev Neurosci 41:61–76
Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ, Chen T, Frohman MA et al (2015) Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 88(5):941–956
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31(7):361–370
Raabe, F.J., L. Slapakova, M.J. Rossner, L. Cantuti-Castelvetri, M. Simons, P.G. Falkai, and A. Schmitt (2019) Oligodendrocytes as a new therapeutic target in schizophrenia: from histopathological findings to neuron-oligodendrocyte interaction. Cells, 8(12).
Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, Chan JR (2012) A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods 9(9):917–922
Bonetto G, Kamen Y, Evans KA, Karadottir RT (2020) Unraveling myelin plasticity Front Cell Neurosci 14:156
Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C (1996) Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A 93(18):9887–9892
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE et al (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344(6183):1252304
Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL et al (2018) Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat Commun 9(1):306
Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, Edgar JM, Yagensky O et al (2017) A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination. Nat Neurosci 20(1):10–15
Siddoway B, Hou H, Yang J, Sun L, Yang H, Wang GY, Xia H (2014) Potassium channel Kv2.1 is regulated through protein phosphatase-1 in response to increases in synaptic activity. Neurosci Lett 583:142–7
Shrager P, Youngman M (2017) Preferential conduction block of myelinated axons by nitric oxide. J Neurosci Res 95(7):1402–1414
Acknowledgements
We thank the technical help of Karen Bentley, director of Electron Microscopy Core of URMC, and Drs. Mathieu Bollen and Aleyde Van Eynde (KU Leuven, Belgium) for providing the floxed NIPP1 mice in our study.
Funding
This work is supported by the National Institutes of Health (NIH) (R01 MH109719) and the National Science Foundation (NSF) (IOS-1457336) to HX and the NIH (F30 MH122046) to KF.
Author information
Authors and Affiliations
Contributions
Houhui Xia contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Cody McKee, Peter Shrager, Arindam Gosh Mazumder, Archan Ganguly, Abigail Mayer, Karl Foley, Nancy Ward, Margaret Youngman, and Hailong Hou. The first draft of the manuscript was written by Houhui Xia and Cody McKee, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All mouse experiments were performed in accordance with protocols approved by the University Committee on Animal Resources of the University of Rochester Medical Center (URMC). Our experiments comply with the ARRIVE guidelines and were carried out in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Research Involving Human Participants and/or Animals
Our research involved use of animals, but not of human subjects.
Informed Consent
Not applicable.
Consent to Participate
Not applicable. There are no human subjects involved in our study.
Consent to Publish
Not applicable. There are no human subjects involved in our study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McKee, C., Shrager, P., Mazumder, A.G. et al. Nuclear Inhibitor of Protein Phosphatase 1 (NIPP1) Regulates CNS Tau Phosphorylation and Myelination During Development. Mol Neurobiol 59, 7486–7494 (2022). https://doi.org/10.1007/s12035-022-03040-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03040-w