Abstract
Introduction
Despite possible risks of mania switching with the long-term use of antidepressants in patients with bipolar disorder (BD), these drugs may help in depressive episodes. Alterations in neurotrophic factor levels seem to be involved in the pathophysiology of BD. The present study aimed to evaluate the effect of acute treatment of imipramine on behavior and neurotrophic levels in rats submitted to the animal model for BD induced by ouabain.
Methods
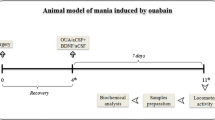
Wistar rats received a single intracerebroventricular (ICV) injection of artificial cerebrospinal fluid or ouabain (10−3 M). Following the ICV administration, the rats were treated for 14 days with saline (NaCl 0.9%, i.p.), lithium (47.5 mg/kg, i.p.), or valproate (200 mg/kg, i.p.). On the 13th and 14th days of treatment, the animals received an additional injection of saline or imipramine (10 mg/kg, i.p.). Behavior tests were evaluated 7 and 14 days after ICV injection. Adrenal gland weight and concentrations of ACTH were evaluated. Levels of neurotrophins BDNF, NGF, NT-3, and GDNF were measured in the frontal cortex and hippocampus by ELISA test.
Results
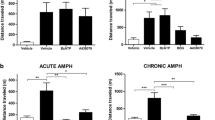
The administration of ouabain induced mania- and depressive-like behavior in the animals 7 and 14 days after ICV, respectively. The treatment with lithium and valproate reversed the mania-like behavior. All treatments were able to reverse most of the depressive-like behaviors induced by ouabain. Moreover, ouabain increased HPA-axis parameters in serum and decreased the neurotrophin levels in the frontal cortex and hippocampus. All treatments, except imipramine, reversed these alterations.
Conclusion
It can be suggested that acute administration of imipramine alone can be effective on depressive-like symptoms but not on neurotrophic factor alterations present in BD.










Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in the manuscript.
Code Availability
Not applicable.
References
Smith DJ, Whitham EA, Ghaemi SN (2012) Bipolar disorder. Handb Clin Neurol 106:251–263. https://doi.org/10.1016/B978-0-444-52002-9.00015-2
Miller JN, Black DW (2020) Bipolar disorder and suicide: a review. Curr Psychiatry Rep 22(2):6. https://doi.org/10.1007/s11920-020-1130-0
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), 5th edn. American Psychiatric Publishing, Washinton (DC)
Wu R, Fan J, Zhao J, Calabrese JR, Gao K (2014) The relationship between neurotrophins and bipolar disorder. Expert Rev Neurother 14(1):51–65. https://doi.org/10.1586/14737175.2014.863709
Gibon J, Barker PA (2017) Neurotrophins and proneurotrophins: focus on synaptic activity and plasticityin the brain. Neuroscientist 23(6):587–604. https://doi.org/10.1177/1073858417697037
Chiou YJ, Huang TL (2019) Brain-derived neurotrophic factor (BDNF) and bipolar disorder. Psychiatry Res 274:395–399. https://doi.org/10.1016/j.psychres.2019.02.051
Barbosa IG, Huguet RB, Neves FS, Reis HJ, Bauer ME, Janka Z, Palotás A, Teixeira AL (2011) Impaired nerve growth factor homeostasis in patientswith bipolar disorder. World J Biol Psychiatry 12(3):228–32. https://doi.org/10.3109/15622975.2010.518629.
Barbosa IG, Huguet RB, Sousa LP, Abreu MN, Rocha NP, Bauer ME, Carvalho LA, Teixeira AL (2011) Circulating levels of GDNF in bipolar disorder. Neurosci Lett 502(2):103–106. https://doi.org/10.1016/j.neulet.2011.07.031
Tseng PT, Chen YW, Tu KY, Wang HY, Chung W, Wu CK, Hsu SP, Kuo HC, Lin PY (2016) State-dependent increase in the levels of neurotrophin-3 and neurotrophin-4/5 in patients with bipolar disorder: a meta-analysis. J Psychiatr Res 79:86–92. https://doi.org/10.1016/j.jpsychires.2016.05.009
Notaras M, van den Buuse M (2020) Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry 25(10):2251–2274. https://doi.org/10.1038/s41380-019-0639-2
Umeoka EHL, van Leeuwen JMC, Vinkers CH, Joëls M (2021) The role of stress in bipolar disorder. Curr Top Behav Neurosci 48:21–39. https://doi.org/10.1007/7854_2020_151
Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 267(9):1244–52. Erratum in: JAMA 1992 Jul 8;268(2):200.
Belvederi-Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, Arzani C, Masotti M, Respino M, Antonioli M, Vassallo L, Serafini G, Perna G, Pompili M, Amore M (2016) The HPA axis in bipolar disorder: systematic review and meta-analysis. Psychoneuroendocrinology 63:327–342. https://doi.org/10.1016/j.psyneuen.2015.10.014
Licht RW (2012) Lithium: still a major option in the management of bipolar disorder. CNS Neurosci Ther 18(3):219–226. https://doi.org/10.1111/j.1755-5949.2011.00260.x
Won E, Kim YK (2017) An oldie but goodie: lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. Int J Mol Sci 18(12):2679. https://doi.org/10.3390/ijms18122679
Valvassori SS, Dal-Pont GC, Resende WR, Jornada LK, Peterle BR, Machado AG, Farias HR, de Souza CT, Carvalho AF, Quevedo J (2017) Lithium and valproate act on the GSK-3β signaling pathway to reverse manic-like behavior in an animal model of mania induced by ouabain. Neuropharmacology 117:447–459. https://doi.org/10.1016/j.neuropharm.2016.10.015
Garnham J, Munro A, Slaney C, Macdougall M, Passmore M, Duffy A, O’Donovan C, Teehan A, Alda M (2007) Prophylactic treatment response in bipolar disorder: results of a naturalistic observation study. J Affect Disord 104(1–3):185–190. https://doi.org/10.1016/j.jad.2007.03.003
Atmaca M (2009) Valproate and neuroprotective effects for bipolar disorder. Int Rev Psychiatry 21(4):410–413. https://doi.org/10.1080/09540260902962206
Cipriani A, Reid K, Young AH, Macritchie K, Geddes J (2013) Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev 10:CD003196. https://doi.org/10.1002/14651858.CD003196.pub2
Bahji A, Ermacora D, Stephenson C, Hawken ER, Vazquez G (2020) Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta-analysis. J Affect Disord 269:154–184. https://doi.org/10.1016/j.jad.2020.03.030
Ball JR, Kiloh LG (1959) A controlled trial of imipramine in treatment of depressive states. Br Med J 2(5159):1052–1055. https://doi.org/10.1136/bmj.2.5159.1052
Romanova EV, Sweedler JV (2018) Animal model systems in neuroscience. ACS Chem Neurosci 9(8):1869–1870. https://doi.org/10.1021/acschemneuro.8b00380
Valvassori SS, Dal-Pont GC, Resende WR, Varela RB, Lopes-Borges J, Cararo JH, Quevedo J (2019) Validation of the animal model of bipolar disorder induced by ouabain: face, construct and predictive perspectives. Transl Psychiatry 9(1):158. https://doi.org/10.1038/s41398-019-0494-6
Looney SW, Mallakh RS (1997) Meta-analysis of erythrocyte Na, K-ATPase activity in bipolar illness. Depress Anxiety 5(2):53–65. https://doi.org/10.1002/(sici)1520-6394(1997)5:2%3c53::aid-da1%3e3.0.co;2-6
Li R, El-Mallakh RS (2004) Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord 80(1):11–17. https://doi.org/10.1016/S0165-0327(03)00044-2
El-Mallakh RS (1983) The Na, K-ATPase hypothesis for manic-depression. I. General considerations. Med Hypotheses 12:253–268
El-Mallakh RS, Wyatt RJ (1995) The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry 37:235–244. https://doi.org/10.1016/0006-3223(94)00201-D
Valvassori SS, Dal-Pont GC, Varela RB, Resende WR, Gava FF, Mina FG, Budni J, Quevedo J (2021) Ouabain induces memory impairment and alter the BDNF signaling pathway in an animal model of bipolar disorder: cognitive and neurochemical alterations in BD model. J Affect Disord 282:1195–1202. https://doi.org/10.1016/j.jad.2020.12.190
Valvassori SS, Cararo JH, Marino CAP, Possamai-Della T, Ferreira CL, Aguiar-Geraldo JM, Dal-Pont GC, Quevedo J (2022) Imipramine induces hyperactivity in rats pretreated with ouabain: Implications to the mania switch induced by antidepressants. J Affect Disord 299:425–434. https://doi.org/10.1016/j.jad.2021.12.021
Allain N, Leven C, Falissard B, Allain JS, Batail JM, Polard E, Montastruc F, Drapier D, Naudet F (2017) Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand 135(2):106–116. https://doi.org/10.1111/acps.12672
Scola G, Andreazza AC (2015) The role of neurotrophins in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 56:122–128. https://doi.org/10.1016/j.pnpbp.2014.08.013
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals, 8th edition. https://www.ncbi.nlm.nih.gov/books/NBK54050/. Accessed 19 Oct 2020
Paxinos G, Watson C (2013) The rat brain in stereotaxic coordinates: hard cover. Academic Press, Cambridge
Jornada LK, Moretti M, Valvassori SS, Ferreira CL, Padilha PT, Arent CO, Fries GR, Kapczinski F, Quevedo J (2010) Effects of mood stabilizers on hippocampus and amygdala BDNF levels in an animal model of mania induced by ouabain. J Psychiatr Res 44(8):506–510. https://doi.org/10.1016/j.jpsychires.2009.11.002
Broadhurst PL (1960) Experiments in psychogenetics: application of biometrical genetics to the inheritance of behavior. In: Eisenk HJ (ed) Experiments in Personality: Psychogenetics and psychopharmacology. Routledge & Kegan Paul, London, pp 31–71
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229(2):327–336
Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C (2003) Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int 42:107–114
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83(2):346–346
Akagawa K, Watanabe M, Tsukada Y (1980) Activity of erythrocyte Na, K-ATPase in manic patients. J Neurochem 35(1):258–260. https://doi.org/10.1111/j.1471-4159.1980.tb12513.x
Hodes A, Rosen H, Cohen-Ben Ami H, Lichtstein D (2019) Na+, K+-ATPase α3 isoform in frontal cortex GABAergic neurons in psychiatric diseases. J Psychiatr Res 115:21–28. https://doi.org/10.1016/j.jpsychires.2019.04.014
Singh SV, Fedorova OV, Wei W, Rosen H, Horesh N, Ilani A, Lichtstein D (2020) Na+, K+-ATPase α isoforms and endogenous cardiac steroids in prefrontal cortex of bipolar patients and controls. Int J Mol Sci 21(16):5912. https://doi.org/10.3390/ijms21165912
Valvassori SS, Resende WR, Lopes-Borges J, Mariot E, Dal-Pont GC, Vitto MF, Luz G, de Souza CT, Quevedo J (2015) Effects of mood stabilizers on oxidative stress-induced cell death signaling pathways in the brains of rats subjected to the ouabain-induced animal model of mania: mood stabilizers exert protective effects against ouabain-induced activation of the cell death pathway. J Psychiatr Res 65:63–70. https://doi.org/10.1016/j.jpsychires.2015.04.009
Varela RB, Resende WR, Dal-Pont GC, Gava FF, Tye SJ, Quevedo J, Valvassori SS (2020) HDAC inhibitors reverse mania-like behavior and modulate epigenetic regulatory enzymes in an animal model of mania induced by ouabain. Pharmacol Biochem Behav 193:172917. https://doi.org/10.1016/j.pbb.2020.172917
Curran G, Ravindran A (2014) Lithium for bipolar disorder: a review of the recent literature. Expert Rev Neurother 14(9):1079–1098. https://doi.org/10.1586/14737175.2014.947965
Nassar A, Azab AN (2014) Effects of lithium on inflammation. ACS Chem Neurosci 5(6):451–8. https://doi.org/10.1021/cn500038f
Tufekci KU, Alural B, Tarakcioglu E, San T, Genc S (2021) Lithium inhibits oxidative stress-induced neuronal senescence through miR-34a. Mol Biol Rep 48(5):4171–4180. https://doi.org/10.1007/s11033-021-06430-w
Barichello T, Milioli G, Generoso JS, Cipriano AL, Costa CS, Moreira AP, Vilela MC, Comim CM, Teixeira AL, Quevedo J (2012) Imipramine reverses depressive-like parameters in pneumococcal meningitis survivor rats. J Neural Transm (Vienna) 119(6):653–660. https://doi.org/10.1007/s00702-011-0749-8
Ceretta LB, Réus GZ, Stringari RB, Ribeiro KF, Zappellini G, Aguiar BW, Pfaffenseller B, Lersh C, Kapczinski F, Quevedo J (2012) Imipramine treatment reverses depressive-like behavior in alloxan-diabetic rats. Diabetes Metab Res Rev 28(2):139–144. https://doi.org/10.1002/dmrr.1285
Wang YJ, Liu L, Wang Y, Wang JL, Gao TT, Wang H, Chen TT, Guan W, Jiang B (2020) Imipramine exerts antidepressant-like effects in chronic stress models of depression by promoting CRTC1 expression in the mPFC. Brain Res Bull 164:257–268. https://doi.org/10.1016/j.brainresbull.2020.08.028
Shyu BC, He AB, Yu YH, Huang ACW (2021) Tricyclic antidepressants and selective serotonin reuptake inhibitors but not anticonvulsants ameliorate pain, anxiety, and depression symptoms in an animal model of central post-stroke pain. Mol Pain 17:17448069211063352. https://doi.org/10.1177/17448069211063351.5
Daban C, Vieta E, Mackin P, Young AH (2005) Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin North Am 28(2):469–480. https://doi.org/10.1016/j.psc.2005.01.005
Valvassori SS, Resende WR, Dal-Pont G, Sangaletti-Pereira H, Gava FF, Peterle BR, Carvalho AF, Varela RB, Dal-Pizzol F, Quevedo J (2017) Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord 19(4):246–258. https://doi.org/10.1111/bdi.12503
Cai L, Mu YR, Liu MM, Tang WJ, Li R (2020) Antidepressant-like effects of penta-acetyl geniposide in chronic unpredictable mild stress-induced depression rat model: Involvement of inhibiting neuroinflammation in prefrontal cortex and regulating hypothalamic-pituitaryadrenal axis. Int Immunopharmacol 80:106182. https://doi.org/10.1016/j.intimp.2019.106182
Hennings JM, Kohli MA, Uhr M, Holsboer F, Ising M, Lucae S (2019) Polymorphisms in the BDNF and BDNFOS genes are associated with hypothalamus-pituitary axis regulation in major depression. Prog Neuropsychopharmacol Biol Psychiatry 95:109686. https://doi.org/10.1016/j.pnpbp.2019.109686
Koshkina A, Dudnichenko T, Baranenko D, Fedotova J, Drago F (2019) Effects of vitamin D3 in long-term ovariectomized rats subjected to chronic unpredictable mild stress: BDNF, NT-3, and NT-4 implications. Nutrients 11(8):1726. https://doi.org/10.3390/nu11081726
Zhao X, Cao F, Liu Q, Li X, Xu G, Liu G, Zhang Y, Yang X, Yi S, Xu F, Fan K, Ma J (2019) Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav Brain Res 364:494–502. https://doi.org/10.1016/j.bbr.2017.05.064
Peng Z, Zhang C, Yan L, Zhang Y, Yang Z, Wang J, Song C (2020) EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippocampal apoptosis signaling in a chronic stress-induced rat model of depression. Int J Mol Sci 21(5):1769. https://doi.org/10.3390/ijms21051769
Chuang DM, Wang Z, Chiu CT (2011) GSK-3 as a target for lithium-induced neuroprotection against excitotoxicity in neuronal cultures and animal models of ischemic stroke. Front Mol Neurosci 4:15. https://doi.org/10.3389/fnmol.2011.00015
Mora E, Portella MJ, Piñol-Ripoll G, López R, Cuadras D, Forcada I, Teres M, Vieta E, Mur M (2019) High BDNF serum levels are associated to good cognitive functioning in bipolar disorder. Eur Psychiatry 60:97–107. https://doi.org/10.1016/j.eurpsy.2019.02.006
Dal-Pont GC, Jório MTS, Resende WR, Gava FF, Aguiar-Geraldo JM, Possamai-Della T, Peper-Nascimento J, Quevedo J, Valvassori SS (2019) Effects of lithium and valproate on behavioral parameters and neurotrophic factor levels in an animal model of mania induced by paradoxical sleep deprivation. J Psychiatr Res 119:76–83. https://doi.org/10.1016/j.jpsychires.2019.09.003
Varela RB, Valvassori SS, Lopes-Borges J, Mariot E, Dal-Pont GC, Amboni RT, Bianchini G, Quevedo J (2015) Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J Psychiatr Res 61:114–121. https://doi.org/10.1016/j.jpsychires.2014.11.003
Segawa M, Morinobu S, Matsumoto T, Fuchikami M, Yamawaki S (2013) Electroconvulsive seizure, but not imipramine, rapidly up-regulates pro-BDNF and t-PA, leading to mature BDNF production, in the rat hippocampus. Int J Neuropsychopharmacol 16(2):339–350. https://doi.org/10.1017/S1461145712000053
Acknowledgements
We would like to acknowledge CNPq, CAPES, FAPESC, and Instituto Cérebro e Mente for the support during the development of the present study.
Funding
Translational Psychiatry Program (USA) is funded by a grant from the National Institute of Health/National Institute of Mental Health (1R21MH117636-01A1, to JQ). Center of Excellence on Mood Disorders (USA) is funded by the Pat Rutherford Jr. Chair in Psychiatry, John S. Dunn Foundation and Anne and Don Fizer Foundation Endowment for Depression Research. Translational Psychiatry Laboratory (Brazil) is funded by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Apoio à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), and Instituto Cérebro e Mente. JQ and SSV are CNPq Research Fellows.
Author information
Authors and Affiliations
Contributions
Samira S. Valvassori and João Quevedo contributed to design and development; methodological design; supervision (responsible for organizing and executing the project); analysis/interpretation and critical review. Jefté Peper-Nascimento, Wilson R. Resende, and Gustavo C. Dal-Pont participated in data collection and processing and biochemical analyses of the samples and performed the statistical analyzes. Taise Possamai-Della and Jorge M. Aguiar-Geraldo performed the statistical analyses and contributed to the analysis/interpretation, literature survey, and writing.
Corresponding author
Ethics declarations
Ethics Approval
The experimental procedures followed the terms of the Brazilian Society for Neuroscience and Behavior (SBNeC), as well as the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The local ethics committee Comissão de Ética no Uso de Animais da Universidade do Extremo Sul Catarinense (protocol number #66/2010) approved this study.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
JQ received clinical research support from LivaNova; has speaker bureau membership with Myriad Neuroscience, Janssen Pharmaceuticals, and Abbvie; is consultant for Eurofarma; is stockholder at Instituto de Neurociencias Dr. Joao Quevedo; and receives copyrights from Artmed Editora, Artmed Panamericana, and Elsevier/Academic Press. All the other authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Possamai-Della, T., Dal-Pont, G.C., Resende, W.R. et al. Imipramine Can Be Effective on Depressive-Like Behaviors, but Not on Neurotrophic Factor Levels in an Animal Model for Bipolar Disorder Induced by Ouabain. Mol Neurobiol 59, 7170–7181 (2022). https://doi.org/10.1007/s12035-022-03022-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03022-y