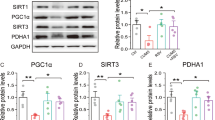
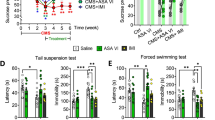
Abstract
Stress-induced neuroinflammation is a hallmark of modern society and has been linked to various emotional disorders, including anxiety. However, how microglia-associated neuroinflammation under chronic unpredictable mild stress (CUMS) alters mitochondrial function and subsequent medial prefrontal cortex-hippocampus (mPFC-HIPP) connectivity remains obscure. We speculated that CUMS might induce neuroinflammation, which involves altered mitochondrial protein levels, blockade of neuroinflammation by a microglial modulator, minocycline, protects against CUMS-induced alterations. Mice were exposed to CUMS for 3 weeks and received minocycline (50 mg/kg) intraperitoneally for 7 consecutive days during the 3rd week of CUMS. Novelty-suppressed feeding test and contextual anxiety test assessed anxiety-like behavior. Western blotting and immunofluorescent staining were employed to evaluate levels of proteins involved in neuroinflammation and mitochondrial function. In vivo dual-site extracellular recordings of local field potential (LFP) were conducted to evaluate the oscillatory activity and brain connectivity in mPFC-HIPP circuitry. We show that CUMS results in excessive microglial activation accompanied by aberrant levels of mitochondrial proteins, such as ATP-5A and the fission protein, Drp-1, increased oxidative stress indicated by elevated levels of nitrotyrosine, and decreased Nrf-2 levels. Furthermore, CUMS causes downregulation of α1 subunit of GABAAR, vesicular GABA transporter (Vgat), and glutamine synthetase (GS), leading to impaired LFP and connectivity of the mPFC-HIPP circuitry. Strikingly, blockage of microglial activation by minocycline ameliorates CUMS-induced aberrant levels of mitochondrial and GABAergic signaling proteins and prevents CUMS-induced anxiety-like behavior in mice. To the end, the study revealed that microglia is critically involved in stress-induced neuroinflammation, which may underlie the molecular mechanism of CUMS-induced anxiety behavior.










Similar content being viewed by others
Data Availability
All data generated or analyzed in this study are available from the corresponding author on reasonable request.
References
Bandelow B, Michaelis S (2015) Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci 17(3):327–335
Disease GBD, Injury I, Prevalence C (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7
Chen HJ, Antonson AM, Rajasekera TA, Patterson JM, Bailey MT, Gur TL (2020) Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms. Transl Psychiatry 10(1):191. https://doi.org/10.1038/s41398-020-00876-5
Soder E, Krkovic K, Lincoln TM (2020) The relevance of chronic stress for the acute stress reaction in people at elevated risk for psychosis. Psychoneuroendocrinology 119:104684. https://doi.org/10.1016/j.psyneuen.2020.104684
Bollinger JL, Horchar MJ, Wohleb ES (2020) Diazepam limits microglia-mediated neuronal remodeling in the prefrontal cortex and associated behavioral consequences following chronic unpredictable stress. Neuropsychopharmacol : Offi Publ Am Coll Neuropsychopharmacol 45(10):1766–1776. https://doi.org/10.1038/s41386-020-0720-1
Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R (2018) Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559(7712):98–102. https://doi.org/10.1038/s41586-018-0262-4
Wang YL, Wu HR, Zhang SS, Xiao HL, Yu J, Ma YY, Zhang YD, Liu Q (2021) Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation. Transl Psychiatry 11(1):353. https://doi.org/10.1038/s41398-021-01468-7
Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA (2008) Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol 33(2):320–331. https://doi.org/10.1038/sj.npp.1301410
Han X, Shao W, Liu Z, Fan S, Yu J, Chen J, Qiao R, Zhou J, Xie P (2015) iTRAQ-based quantitative analysis of hippocampal postsynaptic density-associated proteins in a rat chronic mild stress model of depression. Neuroscience 298:220–292. https://doi.org/10.1016/j.neuroscience.2015.04.006
Goshen I, Yirmiya R (2009) Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 30(1):30–45. https://doi.org/10.1016/j.yfrne.2008.10.001
Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77(1):10–18. https://doi.org/10.1016/j.neuron.2012.12.023
Hafizi S, Da Silva T, Gerritsen C, Kiang M, Bagby RM, Prce I, Wilson AA, Houle S et al (2017) Imaging microglial activation in individuals at clinical high risk for psychosis: an in vivo PET study with [(18)F]FEPPA. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol 42(13):2474–2481. https://doi.org/10.1038/npp.2017.111
Kalin NH (2017) Mechanisms underlying the early risk to develop anxiety and depression: a translational approach. Eur neuropsychopharmacol : j Eur Coll Neuropsychopharmacol 27(6):543–553. https://doi.org/10.1016/j.euroneuro.2017.03.004
Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R (2014) Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 19(6):699–709. https://doi.org/10.1038/mp.2013.155
Feng XY, Hu HD, Chen J, Long C, Yang L, Wang L (2021) Acute neuroinflammation increases excitability of prefrontal parvalbumin interneurons and their functional recruitment during novel object recognition. Brain Behav Immun 98:48–58. https://doi.org/10.1016/j.bbi.2021.08.216
Maes M, Galecki P, Chang YS, Berk M (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 35(3):676–692. https://doi.org/10.1016/j.pnpbp.2010.05.004
Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, Seifert EL, McEwen BS et al (2015) Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc Natl Acad Sci USA 112(48):E6614-6623. https://doi.org/10.1073/pnas.1515733112
Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP (2007) Mitochondria as key components of the stress response. Trends Endocrinol Metab 18(5):190–198. https://doi.org/10.1016/j.tem.2007.04.004
Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O’Neil A, Hayley AC, Pasco JA, Anderson G, Jacka FN, Maes M (2014) Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev 45:46–62. https://doi.org/10.1016/j.neubiorev.2014.05.007
Wilkins HM, Swerdlow RH (2016) Relationships between mitochondria and neuroinflammation: implications for Alzheimer’s disease. Curr Top Med Chem 16(8):849–857. https://doi.org/10.2174/1568026615666150827095102
McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87(3):873–904. https://doi.org/10.1152/physrev.00041.2006
Mateus-Pinheiro A, Patricio P, Alves ND, Martins-Macedo J, Caetano I, Silveira-Rosa T, Araujo B, Mateus-Pinheiro M et al (2021) Hippocampal cytogenesis abrogation impairs inter-regional communication between the hippocampus and prefrontal cortex and promotes the time-dependent manifestation of emotional and cognitive deficits. Mol Psychiatry. https://doi.org/10.1038/s41380-021-01287-8
Arnsten AF (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10(6):410–422. https://doi.org/10.1038/nrn2648
Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA (2016) Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89(4):857–866. https://doi.org/10.1016/j.neuron.2016.01.011
Akam T, Kullmann DM (2014) Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nat Rev Neurosci 15(2):111–122. https://doi.org/10.1038/nrn3668
Adhikari A, Topiwala MA, Gordon JA (2010) Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65(2):257–269. https://doi.org/10.1016/j.neuron.2009.12.002
Han YY, Jin K, Pan QS, Li B, Wu ZQ, Gan L, Yang L, Long C (2020) Microglial activation in the dorsal striatum participates in anxiety-like behavior in Cyld knockout mice. Brain Behav Immun 89:326–338. https://doi.org/10.1016/j.bbi.2020.07.011
Frisbee JC, Brooks SD, Stanley SC, d'Audiffret AC (2015) An unpredictable chronic mild stress protocol for instigating depressive symptoms, behavioral changes and negative health outcomes in rodents. J Vis Exp : JoVE (106) https://doi.org/10.3791/53109
Cerniauskas I, Winterer J, de Jong JW, Lukacsovich D, Yang H, Khan F, Peck JR, Obayashi SK et al (2019) Chronic stress induces activity, synaptic, and transcriptional remodeling of the lateral habenula associated with deficits in motivated behaviors. Neuron 104(5):899-915 e898
Shi X, Gao Y, Song L, Zhao P, Zhang Y, Ding Y, Sun R, Du Y et al (2020) Sulfur dioxide derivatives produce antidepressant- and anxiolytic-like effects in mice. Neuropharmacology 176:108252. https://doi.org/10.1016/j.neuropharm.2020.108252
Li HD, Li DN, Yang L, Long C (2021) Deficiency of the CYLD impairs fear memory of mice and disrupts neuronal activity and synaptic transmission in the basolateral amygdala. Front Cell Neurosci 15:740165. https://doi.org/10.3389/fncel.2021.740165
Luyck K, Tambuyzer T, Deprez M, Rangarajan J, Nuttin B, Luyten L (2017) Electrical stimulation of the bed nucleus of the stria terminalis reduces anxiety in a rat model. Transl Psychiatry 7(2):e1033. https://doi.org/10.1038/tp.2017.2
Luyten L, Casteels C, Vansteenwegen D, van Kuyck K, Koole M, Van Laere K, Nuttin B (2012) Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. J Neurosci : Off J Soc Neurosci 32(1):254–263. https://doi.org/10.1523/JNEUROSCI.3701-11.2012
Steenland HW, Wu V, Fukushima H, Kida S, Zhuo M (2010) CaMKIV over-expression boosts cortical 4–7 Hz oscillations during learning and 1–4 Hz delta oscillations during sleep. Mol Brain 3:16. https://doi.org/10.1186/1756-6606-3-16
Hamrahi H, Chan B, Horner RL (2001) On-line detection of sleep-wake states and application to produce intermittent hypoxia only in sleep in rats. J Appl Physiol 90(6):2130–2140. https://doi.org/10.1152/jappl.2001.90.6.2130
Antzoulatos EG, Miller EK (2014) Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron 83(1):216–225. https://doi.org/10.1016/j.neuron.2014.05.005
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134(1):9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM (2004) The promise of minocycline in neurology. Lancet Neurol 3(12):744–751. https://doi.org/10.1016/S1474-4422(04)00937-8
Garrido-Mesa N, Zarzuelo A, Galvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169(2):337–352. https://doi.org/10.1111/bph.12139
Zhang J, Boska M, Zheng Y, Liu J, Fox HS, Xiong H (2021) Minocycline attenuation of rat corpus callosum abnormality mediated by low-dose lipopolysaccharide-induced microglia activation. J Neuroinflammation 18(1):100. https://doi.org/10.1186/s12974-021-02142-x
Taylor M, Murphy SE, Selvaraj S, Wylezinkska M, Jezzard P, Cowen PJ, Evans J (2008) Differential effects of citalopram and reboxetine on cortical Glx measured with proton MR spectroscopy. J Psychopharmacol 22(5):473–476. https://doi.org/10.1177/0269881107081510
Rajkowska G, Miguel-Hidalgo JJ (2007) Gliogenesis and glial pathology in depression. CNS Neurol Disord: Drug Targets 6(3):219–233. https://doi.org/10.2174/187152707780619326
Guo X, Rao Y, Mao R, Cui L, Fang Y (2020) Common cellular and molecular mechanisms and interactions between microglial activation and aberrant neuroplasticity in depression. Neuropharmacology 181:108336. https://doi.org/10.1016/j.neuropharm.2020.108336
Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17(1):49–59. https://doi.org/10.1038/nri.2016.123
Halder N, Lal G (2021) Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front Immunol 12:660342. https://doi.org/10.3389/fimmu.2021.660342
Li L, Liu Z, Jiang YY, Shen WX, Peng YP, Qiu YH (2019) Acetylcholine suppresses microglial inflammatory response via alpha7nAChR to protect hippocampal neurons. J Integr Neurosci 18(1):51–56. https://doi.org/10.31083/j.jin.2019.01.114
Gamage R, Wagnon I, Rossetti I, Childs R, Niedermayer G, Chesworth R, Gyengesi E (2020) Cholinergic modulation of glial function during aging and chronic neuroinflammation. Front Cell Neurosci 14:577912. https://doi.org/10.3389/fncel.2020.577912
Tata AM, Velluto L, D’Angelo C, Reale M (2014) Cholinergic system dysfunction and neurodegenerative diseases: cause or effect? CNS Neurol Disord: Drug Targets 13(7):1294–1303. https://doi.org/10.2174/1871527313666140917121132
Chang EH, Chavan SS, Pavlov VA (2019) Cholinergic control of inflammation, metabolic dysfunction, and cognitive impairment in obesity-associated disorders: mechanisms and novel therapeutic opportunities. Front Neurosci 13:263. https://doi.org/10.3389/fnins.2019.00263
Zaghloul N, Addorisio ME, Silverman HA, Patel HL, Valdes-Ferrer SI, Ayasolla KR, Lehner KR, Olofsson PS et al (2017) Forebrain cholinergic dysfunction and systemic and brain inflammation in murine sepsis survivors. Front Immunol 8:1673. https://doi.org/10.3389/fimmu.2017.01673
D’Angelo C, Costantini E, Salvador N, Marchioni M, Di Nicola M, Greig NH, Reale M (2021) nAChRs gene expression and neuroinflammation in APPswe/PS1dE9 transgenic mouse. Sci Rep 11(1):9711. https://doi.org/10.1038/s41598-021-89139-x
Candas D, Li JJ (2014) MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal 20(10):1599–1617. https://doi.org/10.1089/ars.2013.5305
Dominiak K, Koziel A, Jarmuszkiewicz W (2018) The interplay between mitochondrial reactive oxygen species formation and the coenzyme Q reduction level. Redox Biol 18:256–265. https://doi.org/10.1016/j.redox.2018.07.018
Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL et al (2015) Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal 23(14):1144–1170. https://doi.org/10.1089/ars.2015.6317
Souza JM, Peluffo G, Radi R (2008) Protein tyrosine nitration—functional alteration or just a biomarker? Free Radical Biol Med 45(4):357–366. https://doi.org/10.1016/j.freeradbiomed.2008.04.010
Hamilton CA, Good AG, Taylor GJ (2001) Induction of vacuolar ATPase and mitochondrial ATP synthase by aluminum in an aluminum-resistant cultivar of wheat. Plant Physiol 125(4):2068–2077. https://doi.org/10.1104/pp.125.4.2068
Lee JE, Westrate LM, Wu H, Page C, Voeltz GK (2016) Multiple dynamin family members collaborate to drive mitochondrial division. Nature 540(7631):139–143. https://doi.org/10.1038/nature20555
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456(7222):605–610. https://doi.org/10.1038/nature07534
Wang J, Zhang W, Lv C, Wang Y, Ma B, Zhang H, Fan Z, Li M et al (2020) A novel biscoumarin compound ameliorates cerebral ischemia reperfusion-induced mitochondrial oxidative injury via Nrf2/Keap1/ARE signaling. Neuropharmacology 167:107918. https://doi.org/10.1016/j.neuropharm.2019.107918
Mah L, Szabuniewicz C, Fiocco AJ (2016) Can anxiety damage the brain? Curr Opin Psychiatry 29(1):56–63. https://doi.org/10.1097/YCO.0000000000000223
Lozano-Soldevilla D, ter Huurne N, Cools R, Jensen O (2014) GABAergic modulation of visual gamma and alpha oscillations and its consequences for working memory performance. Current biol : CB 24(24):2878–2887. https://doi.org/10.1016/j.cub.2014.10.017
Jiang J, Tang B, Wang L, Huo Q, Tan S, Misrani A, Han Y, Li H et al (2022) Systemic LPS-induced microglial activation results in increased GABAergic tone: a mechanism of protection against neuroinflammation in the medial prefrontal cortex in mice. Brain Behav Immun 99:53–69. https://doi.org/10.1016/j.bbi.2021.09.017
Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME (2017) Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol 595(6):1929–1945. https://doi.org/10.1113/JP272134
Albrecht J, Sidoryk-Wegrzynowicz M, Zielinska M, Aschner M (2010) Roles of glutamine in neurotransmission. Neuron Glia Biol 6(4):263–276. https://doi.org/10.1017/S1740925X11000093
Scimemi A (2014) Structure, function, and plasticity of GABA transporters. Front Cell Neurosci 8:161. https://doi.org/10.3389/fncel.2014.00161
Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J (2014) Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15(10):637–654. https://doi.org/10.1038/nrn3819
Morey RA, Haswell CC, Hooper SR, De Bellis MD (2016) Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol 41(3):791–801. https://doi.org/10.1038/npp.2015.205
Kanellopoulos AK, Mariano V, Spinazzi M, Woo YJ, McLean C, Pech U, Li KW, Armstrong JD et al (2020) Aralar sequesters GABA into hyperactive mitochondria, causing social behavior deficits. Cell 180(6):1178-1197 e1120. https://doi.org/10.1016/j.cell.2020.02.044
Camargos QM, Silva BC, Silva DG, Toscano ECB, Oliveira BDS, Bellozi PMQ, Jardim BLO, Vieira ELM et al (2020) Minocycline treatment prevents depression and anxiety-like behaviors and promotes neuroprotection after experimental ischemic stroke. Brain Res Bull 155:1–10. https://doi.org/10.1016/j.brainresbull.2019.11.009
Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M (2014) Minocycline modulates neuropathic pain behaviour and cortical M1–M2 microglial gene expression in a rat model of depression. Brain Behav Immun 42:147–156. https://doi.org/10.1016/j.bbi.2014.06.015
Ohgidani M, Kato TA, Sagata N, Hayakawa K, Shimokawa N, Sato-Kasai M, Kanba S (2016) TNF-alpha from hippocampal microglia induces working memory deficits by acute stress in mice. Brain Behav Immun 55:17–24. https://doi.org/10.1016/j.bbi.2015.08.022
Xu ZX, Kim GH, Tan JW, Riso AE, Sun Y, Xu EY, Liao GY, Xu H et al (2020) Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat Commun 11(1):1797. https://doi.org/10.1038/s41467-020-15530-3
Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H et al (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4:e525. https://doi.org/10.1038/cddis.2013.54
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N et al (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405(6785):458–462. https://doi.org/10.1038/35013070
Lopez-Lopez AL, Jaime HB, Escobar Villanueva MDC, Padilla MB, Palacios GV, Aguilar FJA (2016) Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol Behav 161:15–23. https://doi.org/10.1016/j.physbeh.2016.03.017
Di Meo S, Reed TT, Venditti P, Victor VM (2016) Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev 2016:1245049. https://doi.org/10.1155/2016/1245049
Kang TC (2020) Nuclear factor-erythroid 2-related factor 2 (Nrf2) and mitochondrial dynamics/mitophagy in neurological diseases. Antioxidants 9 (7) https://doi.org/10.3390/antiox9070617
Khalifeh S, Oryan S, Digaleh H, Shaerzadeh F, Khodagholi F, Maghsoudi N, Zarrindast MR (2015) Involvement of Nrf2 in development of anxiety-like behavior by linking Bcl2 to oxidative phosphorylation: estimation in rat hippocampus, amygdala, and prefrontal cortex. Journal of molecular neuroscience : MN 55(2):492–499. https://doi.org/10.1007/s12031-014-0370-z
Kasai S, Shimizu SS Tatara Y, Mimura J, Itoh K (2020) Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 10 (2) https://doi.org/10.3390/biom10020320
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radical Biol Med 88(Pt B):179–188. https://doi.org/10.1016/j.freeradbiomed.2015.04.036
Li Y, Kang C, Wei Z, Qu X, Liu T, Zhou Y, Hu Y (2017) Beta oscillations in major depression—signalling a new cortical circuit for central executive function. Sci Rep 7(1):18021. https://doi.org/10.1038/s41598-017-18306-w
Knyazev GG, Schutter DJ, van Honk J (2006) Anxious apprehension increases coupling of delta and beta oscillations. Int J Psychophysiol 61(2):283–287. https://doi.org/10.1016/j.ijpsycho.2005.12.003
Khemka S, Barnes G, Dolan RJ, Bach DR (2017) Dissecting the function of hippocampal oscillations in a human anxiety model. J Neurosci 37(29):6869–6876. https://doi.org/10.1523/JNEUROSCI.1834-16.2017
Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH et al (2004) Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 28(3):273–283. https://doi.org/10.1016/j.neubiorev.2004.03.004
Bartos M, Vida I, Jonas P (2007) Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8(1):45–56. https://doi.org/10.1038/nrn2044
Whittington MA, Traub RD (2003) Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci 26(12):676–682. https://doi.org/10.1016/j.tins.2003.09.016
David F, Courtiol E, Buonviso N, Fourcaud-Trocme N (2015) Competing mechanisms of gamma and beta oscillations in the olfactory bulb based on multimodal inhibition of mitral cells over a respiratory cycle. eNeuro 2 (6). https://doi.org/10.1523/ENEURO.0018-15.2015
Funding
This study was supported by grants from the National Natural Science Foundation of China (32170950, 31970915, 31871170, 81804197, 31771219), the Guangdong Natural Science Foundation for Major Cultivation Project (2018B030336001), and the Guangdong Grant 'Key Technologies for Treatment of Brain Disorders’ (2018B030332001).
Author information
Authors and Affiliations
Contributions
ST, project initiation, experimental design, western blotting, behavior, statistical analysis, and manuscript writing; AM, western blotting, statistical analysis, and manuscript writing; QH, LFP recording, data analysis, and figure generation; AA, immunostaining and analysis; CL, funding acquisition and supervision; LY guided the experiments and was responsible for funding acquisition and critical revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was approved by the Guangzhou University and South China Normal University Institutional Review Boards. The use of animals in experiments was approved by the Institutional Animal Care and Use Committee (IACUC) and followed National Institutes of Health (NIH) guidelines.
Consent to Participate
Not applicable.
Consent for Publication
All authors have read the manuscript and approved the final version of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tabassum, S., Misrani, A., Huo, Q. et al. Minocycline Ameliorates Chronic Unpredictable Mild Stress-Induced Neuroinflammation and Abnormal mPFC-HIPP Oscillations in Mice. Mol Neurobiol 59, 6874–6895 (2022). https://doi.org/10.1007/s12035-022-03018-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03018-8