Abstract
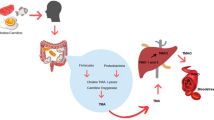
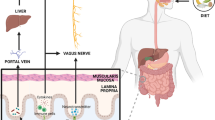
Trimethylamine lyases are expressed in a wide range of intestinal microbiota which metabolize dietary nutrients like choline, betaine, and L-carnitine to form trimethylamine (TMA). Trimethylamine N-oxide (TMAO) is an oxidative product of trimethylamine (TMA) catalyzed by the action of flavin monooxygenases (FMO) in the liver. Higher levels of TMAO in the plasma and cerebrospinal fluid (CSF) have been shown to contribute to the development of risk factors and actively promote the pathogenesis of metabolic, cardiovascular, and cerebrovascular diseases. The investigations on the harmful effects of TMAO in the development and progression of neurodegenerative and sleep disorders are summarized in this manuscript. Clinical investigations on the role of TMAO in predicting risk factors and prognostic factors in patients with neurological disorders are also summarized. It is observed that the mechanisms underlying TMAO-mediated pathogenesis include activation of inflammatory signaling pathways such as nuclear factor kappa B (NF-κβ), NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome, and MAPK/JNK in the periphery and brain. Data suggests that TMAO levels increase with age-related cognitive dysfunction and also induce mitochondrial dysfunction, oxidative stress, neuronal senescence, and synaptic damage in the brain. Further research into the relationships between dietary food consumption and gut microbiota-dependent TMAO levels could provide novel therapeutic options for neurological illnesses.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available in standard research databases such as PubMed, Science Direct, or Google Scholar, and/or on public domains that can be searched with either key words or DOI numbers.
Abbreviations
- Aβ:
-
Amyloid-beta
- AD:
-
Alzheimer’s disease
- ALC:
-
Acetyl L-carnitine
- ALS:
-
Amyotrophic lateral sclerosis
- BBB:
-
Blood-brain barrier
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CVD:
-
Cardiovascular diseases
- EAE:
-
Experimental autoimmune encephalomyelitis
- ETX:
-
Epsilon toxin
- FMO:
-
Flavin-containing monooxygenases
- FMT:
-
Fecal microbiota transplantation
- HD:
-
Huntington’s disease
- JNKs:
-
Jun N-terminal kinases
- LPS:
-
Lipopolysaccharides
- LTP:
-
Long term potentiation
- MAPKs:
-
P38 mitogen-activated protein kinases
- MCI:
-
Mild cognitive impairment
- MS:
-
Multiple sclerosis
- mTOR:
-
Mammalian target of rapamycin
- NF-κβ:
-
Nuclear factor kappa B
- NFTs:
-
Neurofibrillary tangles
- NLRP3:
-
NOD-, LRR-, and pyrin domain-containing protein 3
- OSAS:
-
Obstructive sleep apnea syndrome
- PD:
-
Parkinson’s disease
- PERK:
-
Protein kinase R-like endoplasmic reticulum kinase
- PolyQ:
-
Polyglutamine
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- TDP43:
-
DNA-binding protein 43
- TMA:
-
Trimethylamine
- TMAO:
-
Trimethylamine N-oxide
References
Gregory JC, Buffa JA, Org E et al (2015) Transmission of atherosclerosis susceptibility with gut microbial transplantation *. J Biol Chem 290:5647–5660. https://doi.org/10.1074/JBC.M114.618249
Zhu Y, Jameson E, Crosatti M et al (2014) Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci USA 111:4268–4273. https://doi.org/10.1073/pnas.1316569111
Jie Z, Xia H, Zhong SL et al (2017) The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8:1–11. https://doi.org/10.1038/s41467-017-00900-1
Romano KA, Vivas EI, Amador-noguez D, Rey FE (2015) from diet and accumulation of the proatherogenic metabolite. Intest Microbiota Choline Metab 6:1–8. https://doi.org/10.1128/mBio.02481-14.Editor
Stubbs JR, House JA, Ocque AJ et al (2016) Serum trimethylamine-N-Oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 27:305–313. https://doi.org/10.1681/ASN.2014111063
Koeth RA, Wang Z, Levison BS et al (2013) Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19(5):576–585. https://doi.org/10.1038/nm.3145
Wallace TC, Blusztajn JK, Caudill MA et al (2018) Choline: the underconsumed and underappreciated essential nutrient. Nutr Today 53:240. https://doi.org/10.1097/NT.0000000000000302
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157:121–141. https://doi.org/10.1016/j.cell.2014.03.011
Gagliardi A, Totino V, Cacciotti F, et al (2018) Rebuilding the gut microbiota ecosystem. Int J Environ Res Public Health 15: https://doi.org/10.3390/ijerph15081679
Qin J, Li R, Raes J et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59–65. https://doi.org/10.1038/nature08821
Rinninella E, Raoul P, Cintoni M, et al (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7: https://doi.org/10.3390/MICROORGANISMS7010014
Dinan TG, Cryan JF (2015) The impact of gut microbiota on brain and behaviour: Implications for psychiatry. Curr Opin Clin Nutr Metab Care 18:552–558. https://doi.org/10.1097/MCO.0000000000000221
Eckburg PB, Bik EM, Bernstein CN et al (2005) Microbiology: diversity of the human intestinal microbial flora. Science 308:1635–1638. https://doi.org/10.1126/SCIENCE.1110591/SUPPL_FILE/ECKBURG_SOM.PDF
Bäckhed F, Bäckhed F (2011) Programming of host metabolism by the gut microbiota. Ann Nutr Metab 58:44–52. https://doi.org/10.1159/000328042
Arumugam M, Raes J, Pelletier E et al (2011) Enterotypes of the human gut microbiome. Nature 473:174–180. https://doi.org/10.1038/NATURE09944
Maes M, Kubera M, Leunis JC (2008) The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 29(1):117–24
O’Mahony SM, Marchesi JR, Scully P et al (2009) Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiat 65:263–267. https://doi.org/10.1016/J.BIOPSYCH.2008.06.026
Yun SW, Kim JK, Lee KE et al (2020) A probiotic Lactobacillus gasseri alleviates Escherichia coli-induced cognitive impairment and depression in mice by regulating IL-1β expression and gut microbiota. Nutrients 12:3441. https://doi.org/10.3390/NU12113441
Grenham S, Clarke G, Cryan JF, Dinan TG (2011) Brain-gut-microbe communication in health and disease. Frontiers in Physiology 2 DEC: https://doi.org/10.3389/FPHYS.2011.00094
Clarke G, Grenham S, Scully P et al (2012) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatr 2013 18(6):666–673. https://doi.org/10.1038/mp.2012.77
Sherwin E, Rea K, Dinan TG, Cryan JF (2016) A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol 32:96–102. https://doi.org/10.1097/MOG.0000000000000244
Kelly JR, Borre Y, O’Brien C et al (2016) Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82:109–118. https://doi.org/10.1016/J.JPSYCHIRES.2016.07.019
Chidambaram SB, Essa MM, Rathipriya AG et al (2022) Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: tales of a vicious cycle. Pharmacol Ther 231:107988. https://doi.org/10.1016/J.PHARMTHERA.2021.107988
Ma Q, Xing C, Long W, et al (2019) Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation 16:https://doi.org/10.1186/S12974-019-1434-3
Xiao Q, Shu R, Wu C et al (2020) Crocin-I alleviates the depression-like behaviors probably via modulating “microbiota-gut-brain” axis in mice exposed to chronic restraint stress. J Affect Disord 276:476–486. https://doi.org/10.1016/J.JAD.2020.07.041
Kloiber O, Banjac B, Drewes LR (1988) Protection against acute hyperammonemia: the role of quaternary amines. Toxicology 49:83–90. https://doi.org/10.1016/0300-483X(88)90178-3
Miñana MD, Hermenegildo C, Llansola M et al (1996) Carnitine and choline derivatives containing a trimethylamine group prevent ammonia toxicity in mice and glutamate toxicity in primary cultures of neurons. J Pharmacol Exp Ther 279:194–199
Ma J, Pazos IM, Gai F (2014) Microscopic insights into the protein-stabilizing effect of trimethylamine N-oxide (TMAO). Proc Natl Acad Sci USA 111:8476–8481. https://doi.org/10.1073/pnas.1403224111
Dumas ME, Rothwell AR, Hoyles L et al (2017) Microbial-host co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Rep 20:136–148. https://doi.org/10.1016/J.CELREP.2017.06.039
Wang Z, Klipfell E, Bennett BJ et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472(7341):57–63. https://doi.org/10.1038/nature09922
Vogt NM, Romano KA, Darst BF et al (2018) The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res Ther 10:1–8. https://doi.org/10.1186/s13195-018-0451-2
Gao Q, Wang Y, Wang X et al (2019) Decreased levels of circulating trimethylamine N-oxide alleviate cognitive and pathological deterioration in transgenic mice: a potential therapeutic approach for Alzheimer’s disease. Aging 11:8642–8663. https://doi.org/10.18632/aging.102352
Wang Z, Roberts AB, Buffa JA et al (2015) Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163:1585–1595. https://doi.org/10.1016/J.CELL.2015.11.055
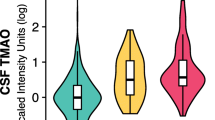
Del Rio D, Zimetti F, Caffarra P et al (2017) The gut microbial metabolite trimethylamine-N-oxide is present in human cerebrospinal fluid. Nutrients 9:1053. https://doi.org/10.3390/NU9101053
Enko D, Zelzer S, Niedrist T et al (2020) Assessment of trimethylamine-n-oxide at the blood-cerebrospinal fluid barrier: results from 290 lumbar punctures. EXCLI J 19:1275–1281. https://doi.org/10.17179/excli2020-2763
Boini KM, Hussain T, Li PL, Koka SS (2017) Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell Physiol Biochem: Int J Exp Cell Physiol, Biochem, Pharmacol 44:152. https://doi.org/10.1159/000484623
Ma GH, Pan B, Chen Y, et al (2017) Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep 37: 10.1042/BSR20160244/82854
Li D, Ke Y, Zhan R et al (2018) Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 17:1–13. https://doi.org/10.1111/acel.12768
Sankowski B, Księżarczyk K, Raćkowska E et al (2020) Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin Chim Acta 501:165–173. https://doi.org/10.1016/j.cca.2019.10.038
Zeisel SH, Warrier M (2017) Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr 37:157–181. https://doi.org/10.1146/annurev-nutr-071816-064732
Bennett BJ, Vallim TQDA, Wang Z et al (2013) Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 17:49–60. https://doi.org/10.1016/j.cmet.2012.12.011
Shih DM, Wang Z, Lee R et al (2015) Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res 56:22–37. https://doi.org/10.1194/JLR.M051680/ATTACHMENT/62754C1E-4EB2-4F8C-9F52-E04A9472A8CF/MMC1.PDF
Li T, Chen Y, Gua C, Li X (2017) Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. 8:1–8. https://doi.org/10.3389/fphys.2017.00350
Janeiro MH, Ramírez MJ, Milagro FI, et al (2018) Implication of trimethylamine n-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients 10: https://doi.org/10.3390/nu10101398
Wang Z, Hazen J, Jia X et al (2021) The nutritional supplement L-alpha glycerylphosphorylcholine promotes atherosclerosis. Int J Mol Sci 22:13477. https://doi.org/10.3390/ijms222413477
Zeisel SH, Mar MH, Howe JC, Holden JM (2003) Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133:1302–1307. https://doi.org/10.1093/JN/133.5.1302
Fennema D, Phillips IR, Shephard EA (2016) Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos 44:1839–1850. https://doi.org/10.1124/DMD.116.070615
Walker JA, Friesen JD, Peters SJ et al (2019) Development of a new and reliable assay for choline kinase using 31P NMR. Heliyon 5:e02585. https://doi.org/10.1016/J.HELIYON.2019.E02585
Koeth RA, Levison BS, Culley MK et al (2014) γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab 20:799–812. https://doi.org/10.1016/J.CMET.2014.10.006
Rebouche CJ, Seim H (1998) Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr 18:39–61. https://doi.org/10.1146/ANNUREV.NUTR.18.1.39
Muramatsu H, Matsuo H, Okada N (2013) Characterization of ergothionase from Burkholderia sp. HME13 and its application to enzymatic quantification of ergothioneine. 5389–5400. https://doi.org/10.1007/s00253-012-4442-0
Craciun S, Balskus EP (2012) Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci USA 109:21307–21312. https://doi.org/10.1073/pnas.1215689109
Al-Waiz M, Mikov M, Mitchell SC, Smith RL (1992) The exogenous origin of trimethylamine in the mouse. Metabolism 41:135–136. https://doi.org/10.1016/0026-0495(92)90140-6
Oellgaard J, Winther SA, Hansen TS, et al (2017) Trimethylamine N-oxide (TMAO) as a new potential therapeutic target for insulin resistance and cancer. Curr Pharm Des 23: https://doi.org/10.2174/1381612823666170622095324
McCrindle SL, Kappler U, McEwan AG (2005) Microbial dimethylsulfoxide and trimethylamine-N-oxide respiration. Adv Microb Physiol 50:147–198. https://doi.org/10.1016/S0065-2911(05)50004-3
Tang WHW, Wang Z, Levison BS et al (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584. https://doi.org/10.1056/NEJMOA1109400
Schmidt AC, Leroux J (2020) Treatments of trimethylaminuria : where we are and where we might be heading. Drug Discovery Today 00: https://doi.org/10.1016/j.drudis.2020.06.026
Mackay RJ, McEntyre CJ, Henderson C et al (2011) Trimethylaminuria: causes and diagnosis of a socially distressing condition. Clin Biochemist Rev 32:33
Tomlinson JAP, Wheeler DC (2017) The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease. Kidney Int 92:809–815. https://doi.org/10.1016/J.KINT.2017.03.053
Velasquez MT, Ramezani A, Manal A, Raj DS (2016) Trimethylamine N-oxide: the good, the bad and the unknown. Toxins 8: https://doi.org/10.3390/toxins8110326
Chhibber-Goel J, Gaur A, Singhal V, et al (2016) The complex metabolism of trimethylamine in humans: endogenous and exogenous sources. Expert Rev Mol Med 18: https://doi.org/10.1017/ERM.2016.6
Taesuwan S, Cho CE, Malysheva OV et al (2017) The metabolic fate of isotopically labeled trimethylamine-N-oxide (TMAO) in humans. J Nutr Biochem 45:77–82. https://doi.org/10.1016/J.JNUTBIO.2017.02.010
Zhang AQ, Mitchell SC, Smith RL (1999) Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol 37:515–520. https://doi.org/10.1016/S0278-6915(99)00028-9
Cho CE, Taesuwan S, Malysheva OV et al (2017) Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res 61:1600324. https://doi.org/10.1002/MNFR.201600324
Gessner A, Di Giuseppe R, Koch M et al (2020) Trimethylamine-N-oxide (TMAO) determined by LC-MS/MS: distribution and correlates in the population-based PopGen cohort. Clin Chem Lab Med 58:733–740. https://doi.org/10.1515/CCLM-2019-1146/DOWNLOADASSET/SUPPL/CCLM-2019-1146_SUPPL.DOCX
Al-Waiz M, Mitchell SC, Idle JR, Smith RL (1987) The metabolism of 14c-labelled trimethylamine and its n-oxide in man. Xenobiotica 17:551–558. https://doi.org/10.3109/00498258709043962
Papandreou C, Moré M, Bellamine A (2020) Trimethylamine N-oxide in relation to cardiometabolic health—cause or effect? Nutrients 12:1330. https://doi.org/10.3390/NU12051330
Boutagy NE, Neilson AP, Osterberg KL et al (2015) Short-term high-fat diet increases postprandial trimethylamine-N-oxide in humans. Nutr Res (New York, NY) 35:858–864. https://doi.org/10.1016/J.NUTRES.2015.07.002
Chen C, Wang C, Hu C et al (2017) Normoalbuminuric diabetic kidney disease. Front Med 11(3):310–318. https://doi.org/10.1007/S11684-017-0542-7
Mohammadi A, Najar AG, Yaghoobi MM et al (2015) Trimethylamine-N-oxide treatment induces changes in the ATP-binding cassette transporter A1 and scavenger receptor A1 in murine macrophage J774A.1 cells. Inflammation 39(1):393–404. https://doi.org/10.1007/S10753-015-0261-7
Mohammadi A, Vahabzadeh Z, Jamalzadeh S, Khalili T (2018) Trimethylamine-N-oxide, as a risk factor for atherosclerosis, induces stress in J774A.1 murine macrophages. Adv Med Sci 63:57–63. https://doi.org/10.1016/J.ADVMS.2017.06.006
Febbraio M, Podrez EA, Smith JD et al (2000) Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Investig 105:1049–1056. https://doi.org/10.1172/JCI9259
Geng J, Yang C, Wang B et al (2018) Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed Pharmacother 97:941–947. https://doi.org/10.1016/J.BIOPHA.2017.11.016
Koka S, Xia M, Chen Y et al (2016) Trimethylamine-N-oxide, an intestinal microbial metabolite instigates NLRP3 inflammasome activation and endothelial dysfunction. FASEB J 30:1204.10-1204.10. https://doi.org/10.1096/FASEBJ.30.1_SUPPLEMENT.1204.10
Sun X, Jiao X, Ma Y et al (2016) Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun 481:63–70. https://doi.org/10.1016/J.BBRC.2016.11.017
Yue C, Yang X, Li J et al (2017) Trimethylamine N-oxide prime NLRP3 inflammasome via inhibiting ATG16L1-induced autophagy in colonic epithelial cells. Biochem Biophys Res Commun 490:541–551. https://doi.org/10.1016/J.BBRC.2017.06.075
Rohrmann S, Linseisen J, Allenspach M et al (2016) Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr 146:283–289. https://doi.org/10.3945/JN.115.220103
Chou RH, Chen CY, Chen IC et al (2019) Trimethylamine N-oxide, circulating endothelial progenitor cells, and endothelial function in patients with stable angina. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-40638-y
Hoyles L, Pontifex MG, Rodriguez-Ramiro I, et al (2021) Regulation of blood–brain barrier integrity and cognition by the microbiome-associated methylamines trimethylamine-N-oxide and trimethylamine. bioRxiv 2021.01.28.428430
Cristante E, Mcarthur S, Mauro C, et al (2013) Identi fi cation of an essential endogenous regulator of blood – brain barrier integrity, and its pathological and therapeutic implications. 110: https://doi.org/10.1073/pnas.1209362110
Vernetti L, Gough A, Baetz N et al (2017) Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci Rep 7:1–14. https://doi.org/10.1038/srep42296
Brunt VE, LaRocca TJ, Bazzoni AE et al (2021) The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 43:377–394. https://doi.org/10.1007/s11357-020-00257-2
Tha KK, Okuma Y, Miyazaki H et al (2000) Changes in expressions of proinflammatory cytokines IL-1β, TNF-α and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res 885:25–31. https://doi.org/10.1016/S0006-8993(00)02883-3
Godbout JP, Johnson RW (2004) Interleukin-6 in the aging brain. J Neuroimmunol 147:141–144. https://doi.org/10.1016/j.jneuroim.2003.10.031
Zhang Y, Zhang C, Li H, Hou J (2019) The presence of high levels of circulating trimethylamine N-oxide exacerbates central and peripheral inflammation and inflammatory hyperalgesia in rats following carrageenan injection. Inflammation 42:2257–2266. https://doi.org/10.1007/s10753-019-01090-2
Ke Y, Li D, Zhao M et al (2018) Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radical Biol Med 116:88–100. https://doi.org/10.1016/j.freeradbiomed.2018.01.007
Csipo T, Lipecz A, Ashpole NM et al (2020) Astrocyte senescence contributes to cognitive decline. GeroScience 42:51–55. https://doi.org/10.1007/s11357-019-00140-9
Jack CR, Albert MS, Knopman DS et al (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dement: J Alzheimer’s Assoc 7:257–262. https://doi.org/10.1016/J.JALZ.2011.03.004
Thompson SM, Kallarackal AJ, Kvarta MD et al (2015) An excitatory synapse hypothesis of depression. Trends Neurosci 38:279–294. https://doi.org/10.1016/J.TINS.2015.03.003
Chidambaram SB, Rathipriya AG, Bolla SR et al (2019) Dendritic spines: revisiting the physiological role. Prog Neuropsychopharmacol Biol Psychiatry 92:161–193. https://doi.org/10.1016/J.PNPBP.2019.01.005
Mucke L (2009) Neuroscience: Alzheimer’s disease. Nature 461:895–897. https://doi.org/10.1038/461895a
Tseng HC, Graves DJ (1998) Natural methylamine osmolytes, trimethylamine N-oxide and betaine, increase tau-induced polymerization of microtubules. Biochem Biophys Res Commun 250:726–730. https://doi.org/10.1006/bbrc.1998.9382
Scaramozzino F, Peterson DW, Farmer P, Gerig JT, Graves DJ, Lew J (2006) TMAO promotes fibrillization and microtubule assembly activity in the C-terminal repeat region of tau. Biochemistry 45(11):3684–3691. https://doi.org/10.1021/bi052167g
MahmoudianDehkordi S, Arnold M, Nho K et al (2019) Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-an emerging role for gut microbiome. Alzheimer’s Dement: J Alzheimer’s Assoc 15:76–92. https://doi.org/10.1016/J.JALZ.2018.07.217
Marizzoni M, Cattaneo A, Mirabelli P et al (2020) Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimer’s Dis: JAD 78:683–697. https://doi.org/10.3233/JAD-200306
Xu R, Wang QQ (2016) Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol 10: https://doi.org/10.1186/s12918-016-0307-y
Harach T, Marungruang N, Duthilleul N, et al (2017) Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 7: https://doi.org/10.1038/srep41802
Govindarajulu M, Pinky PD, Steinke I et al (2020) Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front Mol Neurosci 13:1–13. https://doi.org/10.3389/fnmol.2020.00138
Carrasquillo MM, Belbin O, Hunter TA et al (2010) Replication of CLU, CR1, and PICALM associations with Alzheimer disease. Arch Neurol 67:961–964. https://doi.org/10.1001/archneurol.2010.147
Yu J, Ma X, Wang Y et al (2013) Neurobiology of aging genetic variation in clusterin gene and Alzheimer’s disease risk in Han Chinese. Neurobiol Aging 34:1921.e17-1921.e23. https://doi.org/10.1016/j.neurobiolaging.2013.01.010
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909. https://doi.org/10.1016/S0896-6273(03)00568-3
Schneider SA, Alcalay RN (2017) Neuropathology of genetic synucleinopathies with parkinsonism: review of the literature. Mov Disord: Off J Mov Disord Soc 32:1504–1523. https://doi.org/10.1002/MDS.27193
Sekar S, Mani S, Rajamani B et al (2018) Telmisartan ameliorates astroglial and dopaminergic functions in a mouse model of chronic parkinsonism. Neurotox Res 34:597–612. https://doi.org/10.1007/s12640-018-9921-3
Mani S, Sekar S, Barathidasan R et al (2018) Naringenin decreases α-synuclein expression and neuroinflammation in MPTP-induced Parkinson’s disease model in mice. Neurotox Res 33:656–670. https://doi.org/10.1007/s12640-018-9869-3
Ray B, Ramesh G, Verma SR et al (2021) Effects of telmisartan, an AT1 receptor antagonist, on mitochondria-specific genes expression in a mouse MPTP model of Parkinsonism. Front Biosci - Landmark 26:262–271. https://doi.org/10.52586/4942
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139:318–324. https://doi.org/10.1111/JNC.13691
Pellegrini C, Colucci R, Antonioli L et al (2016) Intestinal dysfunction in Parkinson’s disease: lessons learned from translational studies and experimental models. Neurogastroenterol Motil: Off J Eur Gastrointest Motil Soc 28:1781–1791. https://doi.org/10.1111/NMO.12933
Felice VD, Quigley EM, Sullivan AM et al (2016) Microbiota-gut-brain signalling in Parkinson’s disease: implications for non-motor symptoms. Parkinsonism Relat Disord 27:1–8. https://doi.org/10.1016/J.PARKRELDIS.2016.03.012
Uversky VN, Li J, Fink AL (2001) Trimethylamine-N-oxide-induced folding of α-synuclein. FEBS Lett 509:31–35. https://doi.org/10.1016/S0014-5793(01)03121-0
Hsu LJ, Sagara Y, Arroyo A et al (2000) Α-Synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol 157:401–410. https://doi.org/10.1016/S0002-9440(10)64553-1
Scott DA, Tabarean I, Tang Y et al (2010) A pathologic cascade leading to synaptic dysfunction in α-synuclein-induced neurodegeneration. J Neurosci 30:8083–8095. https://doi.org/10.1523/JNEUROSCI.1091-10.2010
Chen ML, Zhu XH, Ran L, et al (2017) Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc 6: https://doi.org/10.1161/JAHA.117.006347
Sampson TR, Debelius JW, Thron T et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469-1480.e12. https://doi.org/10.1016/j.cell.2016.11.018
Chung SJ, Rim JH, Ji D et al (2021) Gut microbiota-derived metabolite trimethylamine N-oxide as a biomarker in early Parkinson’s disease. Nutrition 83:111090. https://doi.org/10.1016/j.nut.2020.111090
Chen SJ, Kuo CH, Kuo HC et al (2020) The gut metabolite trimethylamine N-oxide is associated with Parkinson’s disease severity and progression. Mov Disord 35:2115–2116. https://doi.org/10.1002/mds.28246
Chu CQ, Yu LL, Chen W et al (2021) Dietary patterns affect Parkinson’s disease via the microbiota-gut-brain axis. Trends Food Sci Technol 116:90–101. https://doi.org/10.1016/J.TIFS.2021.07.004
Brown RH, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. 377:162–172. https://doi.org/10.1056/NEJMRA1603471
Gordon PH (2011) Amyotrophic lateral sclerosis. CNS Drugs 25:1–15. https://doi.org/10.2165/11586000-000000000-00000
Jeon GS, Shim YM, Lee DY et al (2019) Pathological modification of TDP-43 in amyotrophic lateral sclerosis with SOD1 mutations. Mol Neurobiol 56:2007–2021. https://doi.org/10.1007/S12035-018-1218-2/FIGURES/7
Sreedharan J, Blair IP, Tripathi VB et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science (New York, NY) 319:1668–1672. https://doi.org/10.1126/SCIENCE.1154584
Vance C, Rogelj B, Hortobágyi T et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science (New York, NY) 323:1208–1211. https://doi.org/10.1126/SCIENCE.1165942
Longstreth WT, Meschke JS, Davidson SK et al (2005) Hypothesis: a motor neuron toxin produced by a clostridial species residing in gut causes ALS. Med Hypotheses 64:1153–1156. https://doi.org/10.1016/J.MEHY.2004.07.041
Kaneko K, Hachiya NS (2006) Hypothesis: gut as source of motor neuron toxin in the development of ALS. Med Hypotheses 66:438–439. https://doi.org/10.1016/J.MEHY.2005.09.012
Wu S, Yi J, Zhang YG, et al (2015) Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep 3: https://doi.org/10.14814/PHY2.12356
Blacher E, Bashiardes S, Shapiro H et al (2019) Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572:474–480. https://doi.org/10.1038/S41586-019-1443-5
Thrasher JD, Broughton A, Madison R (1990) Immune activation and autoantibodies in humans with long-term inhalation exposure to formaldehyde. Arch Environ Health: Int J 45:217–223. https://doi.org/10.1080/00039896.1990.9940805
Kira Y, Nishikawa M, Ochi A et al (2006) L-Carnitine suppresses the onset of neuromuscular degeneration and increases the life span of mice with familial amyotrophic lateral sclerosis. Brain Res 1070:206–214. https://doi.org/10.1016/j.brainres.2005.11.052
Fang X, Wang X, Yang S et al (2016) Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front Microbiol 7:1–7. https://doi.org/10.3389/fmicb.2016.01479
Rowin J, Xia Y, Jung B, Sun J (2017) Gut inflammation and dysbiosis in human motor neuron disease. Physiol Rep 5:1–6. https://doi.org/10.14814/phy2.13443
Lee A, Arachchige BJ, Reed S et al (2020) Plasma from some patients with amyotrophic lateral sclerosis exhibits elevated formaldehyde levels. J Neurol Sci 409:116589. https://doi.org/10.1016/j.jns.2019.116589
Chen L, Chen Y, Zhao M et al (2020) Changes in the concentrations of trimethylamine N-oxide (TMAO) and its precursors in patients with amyotrophic lateral sclerosis. Sci Rep 10:1–8. https://doi.org/10.1038/s41598-020-72184-3
Getter T, Zaks I, Barhum Y et al (2015) A chemical chaperone-based drug candidate is effective in a mouse model of amyotrophic lateral sclerosis (ALS). ChemMedChem 10:850–861. https://doi.org/10.1002/cmdc.201500045
Beghi E, Pupillo E, Bonito V et al (2013) Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for ALS. Amyotroph Lateral Scler Frontotemporal Degener 14:397–405. https://doi.org/10.3109/21678421.2013.764568
Ross CA, Tabrizi SJ (2011) Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 10:83–98. https://doi.org/10.1016/S1474-4422(10)70245-3
Saravana Babu C, Mahadevan M, Srinivasa Rao B et al (2018) Management of Huntington’s disease: Perspectives from the Siddha system of medicine. Food Huntington’s Dis 159–180
MacDonald ME, Ambrose CM, Duyao MP et al (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983. https://doi.org/10.1016/0092-8674(93)90585-E
Rubinsztein DC, Leggo J, Coles R, et al (1996) Phenotypic characterization of individuals with 30-40 cag repeats in the huntington disease ( HD ) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36-39 Repeats 16–22
Wasser CI, Mercieca E-C, Kong G et al (2020) Gut dysbiosis in Huntington’s disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun 2:1–13. https://doi.org/10.1093/braincomms/fcaa110
Andrich JE, Wobben M, Klotz P et al (2009) Upper gastrointestinal findings in Huntington’s disease: patients suffer but do not complain. J Neural Transm 116(12):1607–1611. https://doi.org/10.1007/S00702-009-0310-1
Van der Burg JMM, Winqvist A, Aziz NA et al (2011) Gastrointestinal dysfunction contributes to weight loss in Huntington’s disease mice. Neurobiol Dis 44:1–8. https://doi.org/10.1016/J.NBD.2011.05.006
Beal MF, Matson WR, Swartz KJ et al (1990) Kynurenine pathway measurements in Huntington’s disease striatum: evidence for reduced formation of kynurenic acid. J Neurochem 55:1327–1339. https://doi.org/10.1111/J.1471-4159.1990.TB03143.X
Verwaest KA, Vu TN, Laukens K et al (2011) 1H NMR based metabolomics of CSF and blood serum: a metabolic profile for a transgenic rat model of Huntington disease. Biochimica et Biophysica Acta (BBA) - Mol Basis Dis 1812:1371–1379. https://doi.org/10.1016/J.BBADIS.2011.08.001
Kong G, Cao KAL, Judd LM, et al (2020) Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington’s disease. Neurobiology of Disease 135:#pagerange#. https://doi.org/10.1016/j.nbd.2018.09.001
Borwankar T, Röthlein C, Zhang G et al (2011) Natural osmolytes remodel the aggregation pathway of mutant huntingtin exon 1. Biochemistry 50:2048–2060. https://doi.org/10.1021/bi1018368
Frischer JM, Bramow S, Dal-bianco A, et al (2009) neurodegeneration in multiple sclerosis brains. https://doi.org/10.1093/brain/awp070
Correale J, Marrodan M, Ysrraelit MC (2019) Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines 7:14. https://doi.org/10.3390/BIOMEDICINES7010014
Vasileiadis GK, Dardiotis E, Mavropoulos A, et al (2018) Regulatory B and T lymphocytes in multiple sclerosis: friends or foes? Auto- immunity highlights 9: https://doi.org/10.1007/S13317-018-0109-X
Constantinescu CS, Farooqi N, O’Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164:1079–1106. https://doi.org/10.1111/J.1476-5381.2011.01302.X
Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. https://doi.org/10.1038/nri3871
Scher JU, Sczesnak A, Longman RS, et al (2013) Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. 1–20. https://doi.org/10.7554/eLife.01202
Kostic AD, Xavier RJ, Gevers D (2014) The microbiome in inflammatory bowel disease : current status. Gastroenterology 146:1489–1499. https://doi.org/10.1053/j.gastro.2014.02.009
Berer K, Mues M, Koutrolos M et al (2011) cooperate to trigger autoimmune demyelination. Nature 479:538–541. https://doi.org/10.1038/nature10554
Rumah KR, Linden J, Fischetti VA, Vartanian T (2013) Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. 8: https://doi.org/10.1371/journal.pone.0076359
Yadav SK, Mindur JE, Ito K, Dhib-jalbut S (2015) Advances in the immunopathogenesis of multiple sclerosis. 1–14. https://doi.org/10.1097/WCO.0000000000000205
Jangi S, Gandhi R, Cox LM, et al (2016) in multiple sclerosis. https://doi.org/10.1038/ncomms12015
Zhao J, Bi W, Xiao S et al (2019) Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-42286-8
Miyake S, Kim S, Suda W, Oshima K (2015) Dysbiosis in the gut microbiota of patients with multiple sclerosis , with a striking depletion of species belonging to Clostridia XIVa and IV clusters. 1–16. https://doi.org/10.1371/journal.pone.0137429
Joossens M, Huys G, Cnockaert M et al (2011) Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 60:631–637. https://doi.org/10.1136/gut.2010.223263
Shahi SK, Freedman SN, Mangalam AK (2017) Gut microbiome in multiple sclerosis : the players involved and the roles they play. 0976: https://doi.org/10.1080/19490976.2017.1349041
Saravana C, Ramanathan M (2011) International Journal of Developmental Neuroscience Post-ischemic administration of nimodipine following focal cerebral ischemic-reperfusion injury in rats alleviated excitotoxicity, neurobehavioural alterations and partially the bioenergetics. Int J Dev Neurosci 29:93–105. https://doi.org/10.1016/j.ijdevneu.2010.08.001
Babu CS, Ramanathan M (2009) Pharmacology, Biochemistry and Behavior Pre-ischemic treatment with memantine reversed the neurochemical and behavioural parameters but not energy metabolites in middle cerebral artery occluded rats. Pharmacol Biochem Behav 92:424–432. https://doi.org/10.1016/j.pbb.2009.01.010
Kim YD, Jung H (2016) Traditional Risk Factors for Stroke in East Asia 18:273–285. https://doi.org/10.5853/jos.2016.00885
Zhu W, Romano KA, Li L et al (2021) Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe 29:1199-1208.e5. https://doi.org/10.1016/j.chom.2021.05.002
Chidambaram SB, Rathipriya AG, Mahalakshmi AM et al (2022) The influence of gut dysbiosis in the pathogenesis and management of ischemic stroke. Cells 11:1239. https://doi.org/10.3390/CELLS11071239
Zhu W, Gregory JC, Org E et al (2016) Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165:111–124. https://doi.org/10.1016/j.cell.2016.02.011
Nie J, Xie L, Zhao BX et al (2018) Serum trimethylamine N-oxide concentration is positively associated with first stroke in hypertensive patients. Stroke 49:2021–2028. https://doi.org/10.1161/STROKEAHA.118.021997
Wu C, Xue F, Lian Y et al (2020) Relationship between elevated plasma trimethylamine N-oxide levels and increased stroke injury. Neurology 94:e667–e677. https://doi.org/10.1212/WNL.0000000000008862
Li XS, Obeid S, Klingenberg R et al (2017) Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 38:814–824. https://doi.org/10.1093/EURHEARTJ/EHW582
Wang Z, Tang WHW, Buffa JA et al (2014) Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 35:904–910. https://doi.org/10.1093/EURHEARTJ/EHU002
Rexidamu M, Li H, Jin H, Huang J (2019) Serum levels of trimethylamine-N-oxide in patients with ischemic stroke. Biosci Rep 39:1–10. https://doi.org/10.1042/BSR20190515
Haghikia A, Li XS, Liman TG et al (2018) Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol 38:2225–2235. https://doi.org/10.1161/ATVBAHA.118.311023
Wu C, Li C, Zhao W et al (2018) Elevated trimethylamine N-oxide related to ischemic brain lesions after carotid artery stenting. Neurology 90:e1283–e1290. https://doi.org/10.1212/WNL.0000000000005298
Farhangi MA, Vajdi M, Asghari-Jafarabadi M (2020) Gut microbiota-associated metabolite trimethylamine N-oxide and the risk of stroke: a systematic review and dose-response meta-Analysis. Nutr J 19: https://doi.org/10.1186/s12937-020-00592-2
Smith RP, Easson C, Lyle SM et al (2019) Gut microbiome diversity is associated with sleep physiology in humans. PLoS One 14:e0222394. https://doi.org/10.1371/JOURNAL.PONE.0222394
Neroni B, Evangelisti M, Radocchia G et al (2021) Relationship between sleep disorders and gut dysbiosis: what affects what? Sleep Med 87:1–7. https://doi.org/10.1016/J.SLEEP.2021.08.003
Coutinho-Wolino KS, Ludmila LFM, de Oliveira LV et al (2021) Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur J Nutr 60:3567–3584. https://doi.org/10.1007/S00394-021-02491-6
Giskeødegård GF, Davies SK, Revell VL, et al (2015) Diurnal rhythms in the human urine metabolome during sleep and total sleep deprivation. Sci Rep 5: https://doi.org/10.1038/SREP14843
Fei N, Choo-Kang C, Reutrakul S et al (2021) Gut microbiota alterations in response to sleep length among African-origin adults. PLoS One 16:1–20. https://doi.org/10.1371/journal.pone.0255323
Mashaqi S, Gozal D (2019) Obstructive sleep apnea and systemic hypertension: Gut dysbiosis as the mediator? J Clin Sleep Med 15:1517–1527. https://doi.org/10.5664/jcsm.7990
Badran M, Mashaqi S, Gozal D (2020) The gut microbiome as a target for adjuvant therapy in obstructive sleep apnea. Expert Opin Ther Targets 24:1263–1282. https://doi.org/10.1080/14728222.2020.1841749
Liu J, Li T, Wu H, et al (2019) Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea-induced hypertension in a high salt diet via regulating TMAO level and CD4 + T cell induced-type I inflammation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 112: https://doi.org/10.1016/J.BIOPHA.2019.01.041
Pak VM, Dai F, Keenan BT et al (2018) Lower plasma choline levels are associated with sleepiness symptoms. Sleep Med 44:89–96. https://doi.org/10.1016/j.sleep.2017.10.004
Moreno-Indias I, Torres M, Montserrat JM et al (2015) Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J 45:1055–1065. https://doi.org/10.1183/09031936.00184314
Durgan DJ, Ganesh BP, Cope JL et al (2016) Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension (Dallas, Tex : 1979) 67:469–474. https://doi.org/10.1161/HYPERTENSIONAHA.115.06672
Acknowledgements
The authors thank their respective institutions for providing the facilities during the manuscript preparation. Figures are drawn using Adobe Photoshop and a few components used in the figures have been adapted and modified from Biorender.com (free trial version).
Funding
This work is supported by the “Public Health and Nutrition Division,” Department of Biotechnology, Ministry of Science and Technology, Govt of India. (BT/PR38038/PFN/20/1528/2020).
Author information
Authors and Affiliations
Contributions
S.S.P., S.S., N.K., H.A.T., C.V., M.K., P.V.K., K.A.C., S.P., A.M.M., J.Y., S.R.P., M.K.S., and S.B.C. made a significant contribution to the work reported, whether that is in the conception, study design, execution, or the acquisition, analysis, or interpretation of data, or all these areas; took part in drafting, revising, or critically reviewing the article; and gave final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors have consented to publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Praveenraj, S.S., Sonali, S., Anand, N. et al. The Role of a Gut Microbial-Derived Metabolite, Trimethylamine N-Oxide (TMAO), in Neurological Disorders. Mol Neurobiol 59, 6684–6700 (2022). https://doi.org/10.1007/s12035-022-02990-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02990-5