Abstract
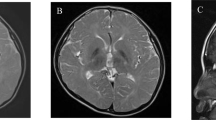
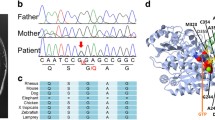
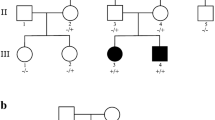
Dominant TUBB4A variants result in different phenotypes, including hypomyelination with atrophy of the basal ganglia and cerebellum (H-ABC), dystonia type 4 (DYT4), and isolated hypomyelination. Here, we report four new patients with a novel TUBB4A variant (p.K324T) and three new patients with previously reported variants (p.Q292K, p.V255I, p.E410K). The individual carrying the novel p.K324T variant exhibits epilepsy of infancy with migrating focal seizures (EIMFS), while the other three have isolated hypomyelination phenotype. We also present a study of the cellular effects of TUBB4A variants responsible for H-ABC (p.D249N), DYT4 (p.R2G), a severe combined phenotype with combination of hypomyelination and EIMFS (p.K324T), and isolated hypomyelination (p.Q292K and p.E410K) on microtubule stability and dynamics, neurite outgrowth, dendritic spine development, and kinesin binding. Cellular-based assays reveal that all variants except p.R2G increase microtubule stability, decrease microtubule polymerization rates, reduce axonal outgrowth, and alter the density and shape of dendritic spines. We also find that the p.K324T and p.E410K variants perturb the binding of TUBB4A to KIF1A, a neuron-specific kinesin required for transport of synaptic vesicle precursors. Taken together, our data suggest that impaired microtubule stability and dynamics, defected axonal growth, and dendritic spine development form the common molecular basis of TUBB4A-related leukodystrophy. Impairment of TUBB4A binding to KIF1A is more likely to be involved in the isolated hypomyelination phenotype, which suggests that alterations in kinesin binding may cause different phenotypes. In conclusion, our study extends the spectrum of TUBB4A mutations and related phenotypes and provides insight into why different TUBB4A variants cause distinct clinical phenotypes.







Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Gierke S, Kumar P, Wittmann T (2010) Analysis of microtubule polymerization dynamics in live cells. Methods Cell Biol 97:15–33. https://doi.org/10.1016/S0091-679X(10)97002-7
Kapitein LC, Hoogenraad CC (2015) Building the neuronal microtubule cytoskeleton. Neuron 87(3):492–506. https://doi.org/10.1016/j.neuron.2015.05.046
Hirokawa N, Noda Y, Tanaka Y, Niwa S (2009) Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 10(10):682–696. https://doi.org/10.1038/nrm2774
Tischfield MA, Engle EC (2010) Distinct alpha- and beta-tubulin isotypes are required for the positioning, differentiation and survival of neurons: new support for the ‘multi-tubulin’ hypothesis. Biosci Rep 30(5):319–330. https://doi.org/10.1042/BSR20100025
Guerrini R, Dobyns WB (2014) Malformations of cortical development: clinical features and genetic causes. Lancet Neurol 13(7):710–726. https://doi.org/10.1016/S1474-4422(14)70040-7
Hirokawa N, Noda Y (2008) Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev 88(3):1089–1118. https://doi.org/10.1152/physrev.00023.2007
Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C et al (2010) Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 140(1):74–87. https://doi.org/10.1016/j.cell.2009.12.011
Sferra A, Fattori F, Rizza T, Flex E, Bellacchio E, Bruselles A, Petrini S, Cecchetti S et al (2018) Defective kinesin binding of TUBB2A causes progressive spastic ataxia syndrome resembling sacsinopathy. Hum Mol Genet 27(11):1892–1904. https://doi.org/10.1093/hmg/ddy096
Lo KY, Kuzmin A, Unger SM, Petersen JD, Silverman MA (2011) KIF1A is the primary anterograde motor protein required for the axonal transport of dense-core vesicles in cultured hippocampal neurons. Neurosci Lett 491(3):168–173. https://doi.org/10.1016/j.neulet.2011.01.018
Niwa S, Takahashi H, Hirokawa N (2013) Beta-tubulin mutations that cause severe neuropathies disrupt axonal transport. EMBO J 32(10):1352–1364. https://doi.org/10.1038/emboj.2013.59
Hersheson J, Mencacci NE, Davis M, MacDonald N, Trabzuni D, Ryten M, Pittman A, Paudel R et al (2013) Mutations in the autoregulatory domain of beta-tubulin 4a cause hereditary dystonia. Ann Neurol 73(4):546–553. https://doi.org/10.1002/ana.23832
Pizzino A, Pierson TM, Guo Y, Helman G, Fortini S, Guerrero K, Saitta S, Murphy JL et al (2014) TUBB4A de novo mutations cause isolated hypomyelination. Neurology 83(10):898–902. https://doi.org/10.1212/WNL.0000000000000754
Miyatake S, Osaka H, Shiina M, Sasaki M, Takanashi J, Haginoya K, Wada T, Morimoto M et al (2014) Expanding the phenotypic spectrum of TUBB4A-associated hypomyelinating leukoencephalopathies. Neurology 82(24):2230–2237. https://doi.org/10.1212/WNL.0000000000000535
Hamilton EM, Polder E, Vanderver A, Naidu S, Schiffmann R, Fisher K, Raguz AB, Blumkin L et al (2014) Hypomyelination with atrophy of the basal ganglia and cerebellum: further delineation of the phenotype and genotype-phenotype correlation. Brain 137(Pt 7):1921–1930. https://doi.org/10.1093/brain/awu110
Duncan ID, Bugiani M, Radcliff AB, Moran JJ, Lopez-Anido C, Duong P, August BK, Wolf NI et al (2017) A mutation in the Tubb4a gene leads to microtubule accumulation with hypomyelination and demyelination. Ann Neurol 81(5):690–702. https://doi.org/10.1002/ana.24930
Curiel J, Rodriguez BG, Takanohashi A, Bugiani M, Fu X, Wolf NI, Nmezi B, Schiffmann R et al (2017) TUBB4A mutations result in specific neuronal and oligodendrocytic defects that closely match clinically distinct phenotypes. Hum Mol Genet 26(22):4506–4518. https://doi.org/10.1093/hmg/ddx338
Vulinovic F, Krajka V, Hausrat TJ, Seibler P, Alvarez-Fischer D, Madoev H, Park JS, Kumar KR et al (2018) Motor protein binding and mitochondrial transport are altered by pathogenic TUBB4A variants. Hum Mutat 39(12):1901–1915. https://doi.org/10.1002/humu.23602
Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK (2011) Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell 22(6):806–816. https://doi.org/10.1091/mbc.E10-03-0269
Tian G, Jaglin XH, Keays DA, Francis F, Chelly J, Cowan NJ (2010) Disease-associated mutations in TUBA1A result in a spectrum of defects in the tubulin folding and heterodimer assembly pathway. Hum Mol Genet 19(18):3599–3613. https://doi.org/10.1093/hmg/ddq276
Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G et al (2003) Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J Neurosci 23(7):2655–2664
van der Knaap MS, Linnankivi T, Paetau A, Feigenbaum A, Wakusawa K, Haginoya K, Kohler W, Henneke M et al (2007) Hypomyelination with atrophy of the basal ganglia and cerebellum: follow-up and pathology. Neurology 69(2):166–171. https://doi.org/10.1212/01.wnl.0000265592.74483.a6
Lohmann K, Wilcox RA, Winkler S, Ramirez A, Rakovic A, Park JS, Arns B, Lohnau T et al (2013) Whispering dysphonia (DYT4 dystonia) is caused by a mutation in the TUBB4 gene. Ann Neurol 73(4):537–545. https://doi.org/10.1002/ana.23829
Ngo L, Haas M, Qu Z, Li SS, Zenker J, Teng KS, Gunnersen JM, Breuss M et al (2014) TUBB5 and its disease-associated mutations influence the terminal differentiation and dendritic spine densities of cerebral cortical neurons. Hum Mol Genet 23(19):5147–5158. https://doi.org/10.1093/hmg/ddu238
Gu J, Firestein BL, Zheng JQ (2008) Microtubules in dendritic spine development. J Neurosci 28(46):12120–12124. https://doi.org/10.1523/JNEUROSCI.2509-08.2008
Hu X, Viesselmann C, Nam S, Merriam E, Dent EW (2008) Activity-dependent dynamic microtubule invasion of dendritic spines. J Neurosci 28(49):13094–13105. https://doi.org/10.1523/JNEUROSCI.3074-08.2008
Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA et al (2009) Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61(1):85–100. https://doi.org/10.1016/j.neuron.2008.11.013
Wong M, Guo D (2013) Dendritic spine pathology in epilepsy: cause or consequence? Neuroscience 251:141–150. https://doi.org/10.1016/j.neuroscience.2012.03.048
Yen TJ, Machlin PS, Cleveland DW (1988) Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature 334(6183):580–585. https://doi.org/10.1038/334580a0
Wade RH (2009) On and around microtubules: an overview. Mol Biotechnol 43(2):177–191. https://doi.org/10.1007/s12033-009-9193-5
Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N (1995) The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81(5):769–780. https://doi.org/10.1016/0092-8674(95)90538-3
Hotchkiss L, Donkervoort S, Leach ME, Mohassel P, Bharucha-Goebel DX, Bradley N, Nguyen D, Hu Y et al (2016) Novel de novo mutations in KIF1A as a cause of hereditary spastic paraplegia with progressive central nervous system involvement. J Child Neurol 31(9):1114–1119. https://doi.org/10.1177/0883073816639718
Lee JR, Srour M, Kim D, Hamdan FF, Lim SH, Brunel-Guitton C, Decarie JC, Rossignol E et al (2015) De novo mutations in the motor domain of KIF1A cause cognitive impairment, spastic paraparesis, axonal neuropathy, and cerebellar atrophy. Hum Mutat 36(1):69–78. https://doi.org/10.1002/humu.22709
Guo Y, Chen Y, Yang M, Xu X, Lin Z, Ma J, Chen H, Hu Y et al (2020) A rare KIF1A missense mutation enhances synaptic function and increases seizure activity. Front Genet 11:61. https://doi.org/10.3389/fgene.2020.00061
Scala M, Bianchi A, Bisulli F, Coppola A, Elia M, Trivisano M, Pruna D, Pippucci T et al (2020) Advances in genetic testing and optimization of clinical management in children and adults with epilepsy. Expert Rev Neurother 20(3):251–269. https://doi.org/10.1080/14737175.2020.1713101
Brouhard GJ, Rice LM (2014) The contribution of alphabeta-tubulin curvature to microtubule dynamics. J Cell Biol 207(3):323–334. https://doi.org/10.1083/jcb.201407095
Fourel G, Boscheron C (2020) Tubulin mutations in neurodevelopmental disorders as a tool to decipher microtubule function. FEBS Lett 594(21):3409–3438. https://doi.org/10.1002/1873-3468.13958
Nogales E, Whittaker M, Milligan RA, Downing KH (1999) High-resolution model of the microtubule. Cell 96(1):79–88. https://doi.org/10.1016/s0092-8674(00)80961-7
Acknowledgements
We are grateful to the patients and their families for their participation in this study. We thank Prof. Jiada Li for providing cell lines and Meng Wu for technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (81771409 and 82071462 to J.P.).
Author information
Authors and Affiliations
Contributions
Conceptualization: H.X. and J.P.; investigation: H.X., F.Y., X.N., and T.W.; resources: J.P.; writing—original draft preparation: H.X.; writing—review and editing: H.H., F.L., and J.P.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Xiangya Hospital of Central South University, China (Human study/protocol #201603205) and performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Written informed consent was obtained from the patients’ parents.
Consent for Publication
The authors affirm that written informed consent for publication was obtained from the patients’ parents.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xiao, H., He, H., Wu, T. et al. Functional Investigation of TUBB4A Variants Associated with Different Clinical Phenotypes. Mol Neurobiol 59, 5056–5069 (2022). https://doi.org/10.1007/s12035-022-02900-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02900-9