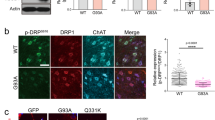
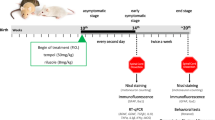
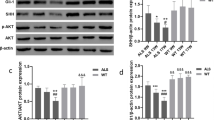
Abstract
Amyotrophic lateral sclerosis (ALS) is the neurodegenerative disease that leads to the motor dysfunction damaged by both upper and lower motor neurons. The etiology and pathogenesis of ALS hasn’t completely been understood yet up to now, the current study suggests that autophagy plays an important role in the development of ALS. Meanwhile, melatonin is found to inhibit the progression of ALS. To this end, this study aimed to investigate the potential relation between melatonin and autophagy in ALS. The in vivo model of ALS was established to investigate the effects of melatonin in ALS. The mRNA expressions were performed to detect by RT-qPCR, and the protein levels were tested by western blot and immunofluorescence histochemistry staining. The inflammatory cytokine was applied to detect by ELISA. The results showed that melatonin dose-dependently reversed the ALS-induced survival time shortened, weight loss and rotating rod latency decrease. The expressions of both SIRT1 and Beclin-1 as well as the ratio of LC3II/LC3I were significantly upregulated in the ALS mice, while melatonin reversed the upregulation of both SIRT1 and Beclin-1 expression and LC3II/LC3I ratio in a dose-dependent manner. In contrast, melatonin dose-dependently significantly restored the ALS-induced downregulation of p62. Furthermore, SIRT1 silencing notably reduced the effect of melatonin on Beclin-1, LC3II/LC3I, and p62. Melatonin induced autophagy in the ALS mice via the upregulation of SIRT1. Thus, melatonin might act as a new agent for the treatment of ALS.






Similar content being viewed by others
Data Aavailability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- AMPK:
-
AMP-activated protein kinase
- ANOVA:
-
Analysis of variance
- Bcl-2:
-
B-cell lymphoma-2
- Bax:
-
Bcl-2-Associated X
- cDNA:
-
Complementary DNA
- DAPI:
-
4',6-Diamidino-2-phenylindole, dihydrochloride
- ELISA:
-
Enzyme-linked immunosorbent assay
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- K-M cure:
-
Kaplan–Meier
- MN:
-
Motoneurons
- PVDF:
-
Poly (vinylidene fluoride)
- RIPA:
-
Radio-Immunoprecipitation Assay
- Rpm:
-
Rotations per minute
- RT-qPCR:
-
Quantitative reverse transcription PCR
- SD:
-
Standard deviation
- siRNA:
-
Small interferring RNA
- SOD1:
-
Superoxide dismutase 1
- TBK1:
-
TANK binding kinase 1
- TG:
-
Transgenic mice expressing human SOD1G93A
- TNF-α:
-
Tumor necrosis factor-α
- WT:
-
Wild type mice
References
Huang X, Roet KCD, Zhang L, Brault A, Berg AP, Jefferson AB, Klug-McLeod J, Leach KL, Vincent F, Yang H, Coyle AJ, Jones LH, Frost D, Wiskow O, Chen K, Maeda R, Grantham A, Dornon MK, Klim JR, Siekmann MT, Zhao D, Lee S, Eggan K, Woolf CJ (2021) Human amyotrophic lateral sclerosis excitability phenotype screen: target discovery and validation. Cell Rep 35(10):109224. https://doi.org/10.1016/j.celrep.2021.109224
Wu R, Zhou D, Shen X, Chen F, Liu F, Gu J (2021) Phosphorylation of trans-active response DNA binding protein-of 43 kDa promotes its cytoplasmic aggregation and modulates its function in tau mRNA stability and exon 10 alternative splicing. J Neurochem 158(3):766–778. https://doi.org/10.1111/jnc.15450
Si Y, Kazamel M, Benatar M, Wuu J, Kwon Y, Kwan T, Jiang N, Kentrup D, Faul C, Alesce L, King PH (2021) FGF23, a novel muscle biomarker detected in the early stages of ALS. Sci Rep 11(1):12062. https://doi.org/10.1038/s41598-021-91496-6
Kulkarni NP, Vaidya B, Narula A, Sharma SS (2021) Neuroprotective potential of caffeic acid phenethyl ester (CAPE) in CNS disorders: mechanistic and therapeutic insights. Curr Neuropharmacol 19(9):1401–1415. https://doi.org/10.2174/1570159X19666210608165509
Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2012(3):CD001447. https://doi.org/10.1002/14651858.CD001447.pub3
Chiò A, Mazzini L, Mora G (2020) Disease-modifying therapies in amyotrophic lateral sclerosis. Neuropharmacology 167:107986. https://doi.org/10.1016/j.neuropharm.2020.107986
Bianchi VE, Locatelli V, Rizzi L (2017) Neurotrophic and neuroregenerative effects of GH/IGF1. Int J Mol Sci 18(11):2441. https://doi.org/10.3390/ijms18112441
Alhindi A, Boehm I, Chaytow H (2021) Small junction, big problems: neuromuscular junction pathology in mouse models of amyotrophic lateral sclerosis (ALS). J Anat. https://doi.org/10.1111/joa.13463
Apolloni S, D’Ambrosi N (2022) Fibrosis as a common trait in amyotrophic lateral sclerosis tissues. Neural Regen Res 17(1):97–98. https://doi.org/10.4103/1673-5374.314302
Zakharova MN, Abramova AA (2022) Lower and upper motor neuron involvement and their impact on disease prognosis in amyotrophic lateral sclerosis. Neural Regen Res 17(1):65–73. https://doi.org/10.4103/1673-5374.314289
Filippini T, Hatch EE, Vinceti M (2021) Residential exposure to electromagnetic fields and risk of amyotrophic lateral sclerosis: a dose-response meta-analysis. Sci Rep 11(1):11939. https://doi.org/10.1038/s41598-021-91349-2
Sun Y, Wang C, Zhang N, Liu F (2021) Melatonin ameliorates hypertension in hypertensive pregnant mice and suppresses the hypertension-induced decrease in Ca(2+)-activated K(+) channels in uterine arteries. Hypertens Res 44(9):1079–1086. https://doi.org/10.1038/s41440-021-00675-5
Struijk C, van der Poel N, Blommaerts I, Boiy T, Hofkens-Van Den Brandt A, Van Den Brande K, Vanderveken O, Vermeersch H, Boudewyns A (2021) The use of melatonin for auditory brainstem response audiometry in children with comorbidities. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-021-06923-1
Ishihara R, Barros MP, Silva CMD, Borges LDS, Hatanaka E, Lambertucci RH (2021) Melatonin improves the antioxidant capacity in cardiac tissue of Wistar rats after exhaustive exercise. Free Radic Res 55(7):776–791. https://doi.org/10.1080/10715762.2021.1939024
Wu Y, He F, Zhang C, Zhang Q, Su X, Zhu X, Liu A, Shi W, Lin W, Jin Z, Yang H, Lin J (2021) Melatonin alleviates titanium nanoparticles induced osteolysis via activation of butyrate/GPR109A signaling pathway. J Nanobiotechnology 19(1):170. https://doi.org/10.1186/s12951-021-00915-3
Bald EM, Nance CS, Schultz JL (2021) Melatonin may slow disease progression in amyotrophic lateral sclerosis: findings from the pooled resource open-access ALS clinic trials database. Muscle Nerve 63(4):572–576. https://doi.org/10.1002/mus.27168
Hofer SJ, Liang Y, Zimmermann A, Schroeder S, Dengjel J, Kroemer G, Eisenberg T, Sigrist SJ, Madeo F (2021) Spermidine-induced hypusination preserves mitochondrial and cognitive function during aging. Autophagy 17(8):2037–2039. https://doi.org/10.1080/15548627.2021.1933299
Ji C, Zhao YG (2021) The BPAN and intellectual disability disease proteins WDR45 and WDR45B modulate autophagosome-lysosome fusion. Autophagy 17(7):1783–1784. https://doi.org/10.1080/15548627.2021.1924039
Liu Y, Bi YM, Pan T, Zeng T, Mo C, Sun B, Gao L, Lyu ZP (2021) Ethyl acetate fraction of Dicliptera chinensis (L.) Juss. ameliorates liver fibrosis by inducing autophagy via PI3K/AKT/mTOR/p70S6K signaling pathway. Chin J Integr Med 28(1):60–68. https://doi.org/10.1007/s11655-021-3298-5
Wang Y, Li Z, Teng M, Liu J (2021) Dihydroartemisinin inhibits activation of the AIM2 inflammasome pathway and NF-kappaB/HIF-1alpha/VEGF pathway by inducing autophagy in A431 human cutaneous squamous cell carcinoma cells. Int J Med Sci 18(12):2705–2715. https://doi.org/10.7150/ijms.57167
Kubicka-Trzaska A, Zuber-Laskawiec K, Plutecka H, Romanowska-Dixon B, Sanak M, Karska-Basta I (2021) Altered serum levels of autophagy proteins Beclin-1 and mTOR in patients with exudative age-related macular degeneration. J Physiol Pharmacol. 72(1). https://doi.org/10.26402/jpp.2021.1.09
Wang Y, Wang P, Zhao L, Chen X, Lin Z, Zhang L, Li Z (2021) miR-224-5p carried by human umbilical cord mesenchymal stem cells-derived exosomes regulates autophagy in breast cancer cells via HOXA5. Front Cell Dev Biol 9:679185. https://doi.org/10.3389/fcell.2021.679185
Chua JP, De Calbiac H, Kabashi E, Barmada SJ (2021) Autophagy and ALS: mechanistic insights and therapeutic implications. Autophagy 18(2):254–282. https://doi.org/10.1080/15548627.2021.1926656
Moujalled D, Strasser A, Liddell JR (2021) Molecular mechanisms of cell death in neurological diseases. Cell Death Differ 28(7):2029–2044. https://doi.org/10.1038/s41418-021-00814-y
Bharathi V, Girdhar A, Patel BK (2021) Role of CNC1 gene in TDP-43 aggregation-induced oxidative stress-mediated cell death in S. cerevisiae model of ALS. Biochim Biophys Acta Mol Cell Res 1868(6):118993. https://doi.org/10.1016/j.bbamcr.2021.118993
Wu K, Seylani A, Wu J, Wu X, Bleck CKE, Sack MN (2021) BLOC1S1/GCN5L1/BORCS1 is a critical mediator for the initiation of autolysosomal tubulation. Autophagy 17(11):3707–3724. https://doi.org/10.1080/15548627.2021.1894759
Xie F, Zhang J, Zhai M, Liu Y, Hu H, Yu Z, Zhang J, Lin S, Liang D, Cao Y (2021) Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-Akt pathway. Reproduction 162(1):73–82. https://doi.org/10.1530/REP-20-0643
Bai Y, Yang Y, Gao Y, Lin D, Wang Z, Ma J (2021) Melatonin postconditioning ameliorates anoxia/reoxygenation injury by regulating mitophagy and mitochondrial dynamics in a SIRT3-dependent manner. Eur J Pharmacol 904:174157. https://doi.org/10.1016/j.ejphar.2021.174157
Li H, Wei EQ, Yang Y (2015) Beclin 1 regulates apoptosis and autophagy. Chin J Biochem Mol Biol 31(4):331–338
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13(4):225–238. https://doi.org/10.1038/nrm3293
Villalba JM, Alcaín FJ (2012) Sirtuin activators and inhibitors. BioFactors 38(5):349–359. https://doi.org/10.1002/biof.1032
Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S (2007) H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem 20(1–4):45–54. https://doi.org/10.1159/000104152
He M, Tan B, Vasan K, Yuan H, Cheng F, da Silva SR, Lu C, Gao SJ (2017) SIRT1 and AMPK pathways are essential for the proliferation and survival of primary effusion lymphoma cells. J Pathol 242(3):309–321. https://doi.org/10.1002/path.4905
Zhao GN, Tian ZW, Tian T, Zhu ZP, Zhao WJ, Tian H, Cheng X, Hu FJ, Hu ML, Tian S, Ding T, Chen S, Ji YX, Zhang P, Zhang XJ, She ZG, Yuan Y, Chen W, Bai L, Li H (2021) TMBIM1 is an inhibitor of adipogenesis and its depletion promotes adipocyte hyperplasia and improves obesity-related metabolic disease. Cell Metab 33(8):1640-1654.e8. https://doi.org/10.1016/j.cmet.2021.05.014
Shao F, Zhou L, Zhang Y, Chen H, Zhang Y, Guan Z (2021) Gastrodin alleviates inflammatory injury of cardiomyocytes in septic shock mice via inhibiting NLRP3 expression. In Vitro Cell Dev Biol Anim 57(5):571–581. https://doi.org/10.1007/s11626-021-00593-3
Rubio-González A, Bermejo-Millo JC, de Luxán-Delgado B, Potes Y, Pérez-Martínez Z, Boga JA, Vega-Naredo I, Caballero B, Solano JJ, Coto-Montes A, Members of Research Team cROS (cellular Response to Oxidative Stress) (2018) Melatonin prevents the harmful effects of obesity on the brain, including at the behavioral level. Mol Neurobiol 55(7):5830–5846. https://doi.org/10.1007/s12035-017-0796-8
Luo F, Sandhu AF, Rungratanawanich W, Williams GE, Akbar M, Zhou S, Song BJ, Wang X (2020) Melatonin and autophagy in aging-related neurodegenerative diseases. Int J Mol Sci 21(19):7174. https://doi.org/10.3390/ijms21197174
Zhang Y, Fan D, Liu X, Liu X, He J, Zhang N, Tang L (2020) hTBK1-c.978T>A mutation promotes the ferroptosis in NSC-34 cells via mediation of KEAP1/NRF2/p62 signaling. Am J Transl Res 12(11):7386–7394
Duan W, Yi L, Tian Y, Huang HP, Li Z, Bi Y, Guo M, Li Y, Liu Y, Ma Y, Song X, Liu Y, Li C (2021) Myeloid TBK1 deficiency induces motor deficits and axon degeneration through inflammatory cell infiltration. Mol Neurobiol 58(5):2435–2446. https://doi.org/10.1007/s12035-020-02235-3
Sieverding K, Ulmer J, Bruno C, Satoh T, Tsao W, Freischmidt A, Akira S, Wong PC, Ludolph AC, Danzer KM, Lobsiger CS, Brenner D, Weishaupt JH (2021) Hemizygous deletion of Tbk1 worsens neuromuscular junction pathology in TDP-43(G298S) transgenic mice. Exp Neurol 335:113496. https://doi.org/10.1016/j.expneurol.2020.113496
Gu C, Wang F, Zhang YT, Wei SZ, Liu JY, Sun HY, Wang GH, Liu CF (2021) Microglial MT1 activation inhibits LPS-induced neuroinflammation via regulation of metabolic reprogramming. Aging Cell e13375. https://doi.org/10.1111/acel.13375
Wang C, Zhao Z, Qi Q, Wang J, Kong Y, Feng Z, Chen A, Li W, Zhang Q, Wang J, Huang B, Li X (2021) miR-6858 plays a key role in the process of melatonin inhibition of the malignant biological behavior of glioma. J Clin Neurosci 87:137–146. https://doi.org/10.1016/j.jocn.2021.02.015
Do HA, Baek KH (2021) Cellular functions regulated by deubiquitinating enzymes in neurodegenerative diseases. Ageing Res Rev 69:101367. https://doi.org/10.1016/j.arr.2021.101367
Su G, Feng T, Pei T, Yang F, Sun D, Yu H, Wang X, Gao W, He J, Shen Y, Liu X (2021) Autophagy modulates FSS-induced epithelial-mesenchymal transition in hepatocellular carcinoma cells. Mol Carcinog 60(9):607–619. https://doi.org/10.1002/mc.23327
Li R, Zhao X, Zhang S, Dong W, Zhang L, Chen Y, Li Z, Yang H, Huang Y, Xie Z, Wang W, Li C, Ye Z, Dong Z, Liang X (2021) RIP3 impedes transcription factor EB to suppress autophagic degradation in septic acute kidney injury. Cell Death Dis 12(6):593. https://doi.org/10.1038/s41419-021-03865-8
Sadria M, Layton AT (2021) Interactions among mTORC, AMPK and SIRT: a computational model for cell energy balance and metabolism. Cell Commun Signal 19(1):57. https://doi.org/10.1186/s12964-021-00706-1
Yang C, Xu X, Dong X, Yang B, Dong W, Luo Y, Liu X, Wu Y (1868) Wang J (2021) DDIT3/CHOP promotes autophagy in chondrocytes via SIRT1-AKT pathway. Biochim Biophys Acta Mol Cell Res 9:119074. https://doi.org/10.1016/j.bbamcr.2021.119074
Liu S, Mok BW, Deng S, Liu H, Wang P, Song W, Chen P, Huang X, Zheng M, Lau SY, Cremin CJ, Tam CY, Li B, Jiang L, Chen Y, Yuen KY, Chen H (2021) Mammalian cells use the autophagy process to restrict avian influenza virus replication. Cell Rep 35(10):109213. https://doi.org/10.1016/j.celrep.2021.109213
Yang Z, Su W, Zhang Y, Zhou L, Xia ZY, Lei S (2021) Selective inhibition of PKCbeta2 improves Caveolin-3/eNOS signaling and attenuates lipopolysaccharide-induced injury by inhibiting autophagy in H9C2 cardiomyocytes. J Mol Histol 52(4):705–715. https://doi.org/10.1007/s10735-021-09990-0
Yoshihisa Y, Rehman MU, Andoh T, Tabuchi Y, Makino T, Shimizu T (2021) Overexpression of D-dopachrome tautomerase increases ultraviolet B irradiation-induced skin tumorigenesis in mice. FASEB J 35(7):e21671. https://doi.org/10.1096/fj.202002631RRR
Ni J, Huang Z, Wang D (2021) LncRNA TP73-AS1 promotes oxidized low-density lipoprotein-induced apoptosis of endothelial cells in atherosclerosis by targeting the miR-654-3p/AKT3 axis. Cell Mol Biol Lett 26(1):27. https://doi.org/10.1186/s11658-021-00264-x
Yao J, Liu X, Sun Y, Dong X, Liu L, Gu H (2021) Curcumin-alleviated osteoarthritic progression in rats fed a high-fat diet by inhibiting apoptosis and activating autophagy via modulation of microRNA-34a. J Inflamm Res 14:2317–2331. https://doi.org/10.2147/JIR.S312139
Zhang H, Yan A, Liu X, Ma Y, Zhao F, Wang M, JLoor JJ, Wang H (2021) Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J Hazard Mater 407:124489. https://doi.org/10.1016/j.jhazmat.2020.124489
Tan DX, Hardeland D (2020) Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 25(19):4410. https://doi.org/10.3390/molecules25194410
Li J, Wang C, Xue L, Zhang F, Liu J (2021) Melatonin suppresses apoptosis of nucleus pulposus cells through inhibiting autophagy via the PI3K/Akt pathway in a high-glucose culture. Biomed Res Int 2021:4604258. https://doi.org/10.1155/2021/4604258
Huang X, Hou J, Huang S, Feng K, Yue Y, Li H, Huang S, Liang M, Chen G, Wu Z (2021) Melatonin ameliorates myocardial injury by reducing apoptosis and autophagy of cardiomyocytes in a rat cardiopulmonary bypass model. Peer J 9:e11264. https://doi.org/10.7717/peerj.11264
Acknowledgements
We gratefully thank all technicians of Changsha Dongsheng Biotechnology Co., Ltd. (Unified social credit Code: 91430105MA4LMDL7XT), Changsha, China and Multisciences (Lianke) Biotech Co., Ltd, Hangzhou, China for performing all experiments and analyzing the rough data and providing the related technology supports. And sincerely acknowledge Prof. Dongyuan Yao at Jiangxi Provincial Neurological Institute for language editing.
Funding
This study was supported by grants from the Committee of National Natural Science Foundation of China (Grant nos. 30560042, 81160161, 81360198 and 82160255), Education Department of Jiangxi Province (Grant nos. GJJ13198 and GJJ170021), Jiangxi provincial department of science and technology (Grant nos. [2014]-47, 20142BBG70062, 20171BAB215022, and 20192BAB205043) and Health and Family Planning Commission of Jiangxi province (Grant no. 20181019).
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the research and preparation of the manuscript. Xiaoping Shen, Chunyan Tang, and Yu Zhu carried out cell culture and animal breed. Xiaoping Shen, Chunyan Tang, Caihui Wei, and Yu Zhu carried out western-blot and behavioral test. Xiaoping Shen, and Renshi Xu were responsible for the experimental design and writing the manuscript. Xiaoping Shen, Chunyan Tang, and Caihui Wei are the common first jointed authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the standards upheld by the local ethics committee of Affiliated Jiujiang Hospital of Nanchang University (Approval No. 2020–9–12). The experiments were carried out strictly in line with the requirements of the International Association for the Study of Pain. All experiments were conducted in accordance with the Guide of the US National Institutes of Health (revised in 1985 [publication No. 85–23]) for the Care and Use of Laboratory Animals.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Melatonin upregulates Beclin-1 and SIRT1 in ALS mice.
• Melatonin increases the survival period of ALS mice.
• Melatonin ameliorates the motor function of ALS mice.
• SIRT1 silencing reverses the therapeutic effect of melatonin on ALS.
Jiangxi Provincial People’s Hospital and Medical College of Nanchang University are the jointed finished unit and equal contribution to this study.
Rights and permissions
About this article
Cite this article
Shen, X., Tang, C., Wei, C. et al. Melatonin Induces Autophagy in Amyotrophic Lateral Sclerosis Mice via Upregulation of SIRT1. Mol Neurobiol 59, 4747–4760 (2022). https://doi.org/10.1007/s12035-022-02875-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02875-7