Abstract
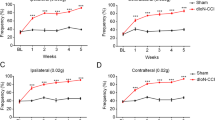
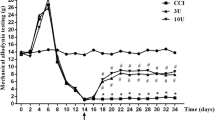
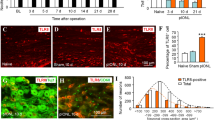
BmK DKK13 (DKK13) is a mutated recombinant peptide, which has a significant antinociception in a rat model of the inflammatory pain. The purpose of this study was to evaluate the antinociceptive effect of DKK13 on trigeminal neuralgia (TN) in rats. Male Sprague–Dawley (SD) rats were treated with the chronic constriction injury of the infraorbital nerve (IoN-CCI) model to induce stable symptoms of TN. DKK13 (1.0 mg/kg, 2.0 mg/kg and 4.0 mg/kg, i.v.) or morphine (4.0 mg/kg, i.v.) was administered by tail vein once on day 14 after IoN-CCI injury. Behavioral tests, electrophysiology and western blotting were performed to investigate the role and underlying mechanisms of DKK13 on IoN-CCI model. Behavioral test results showed that DKK13 could significantly increase the mechanical pain and thermal radiation pain thresholds of IoN-CCI rats and inhibit the asymmetric spontaneous pain scratching behavior. Electrophysiological results showed that DKK13 could significantly reduce the current density of Nav1.8 in the ipsilateral side of trigeminal ganglion (TG) neurons in IoN-CCI rats, and the steady-state activation and inactivation curves of Nav1.8 shifted, respectively, to the direction of hyperpolarization and depolarization. Western blotting results showed that DKK13 significantly reduced the expression of Nav1.8 and the phosphorylation levels of key proteins of MAPKs/CREB pathway in TG tissues of IoN-CCI rats. In brief, DKK13 has a significant antinociceptive effect on IoN-CCI rats, which may be achieved by changing the dynamic characteristics of Nav1.8 channel and regulating the protein phosphorylation in MAPKs/CREB pathway.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Freeman R, Edwards R, Baron R, Bruehl S, Cruccu G, Dworkin RH, Haroutounian S (2019) AAPT Diagnostic Criteria for Peripheral Neuropathic Pain: Focal and Segmental Disorders. J Pain 20:369–393. https://doi.org/10.1016/j.jpain.2018.10.002
Zakrzewska JM, Wu J, Mon-Williams M, Phillips N, Pavitt SH (2017) Evaluating the impact of trigeminal neuralgia. Pain 158:1166–1174. https://doi.org/10.1097/j.pain.0000000000000853
Brzegowy K, Pękala PA, Zarzecki MP, Pękala JR, Roy J, Aziz HM, Tubbs RS, Walocha JA, Tomaszewski KA, Mikos M (2020) Prevalence and Clinical Implications of the Primitive Trigeminal Artery and its Variants: A Meta-Analysis. World Neurosurg 133:e401–e411. https://doi.org/10.1016/j.wneu.2019.09.042
Araya EI, Claudino RF, Piovesan EJ, Chichorro JG (2020) Trigeminal Neuralgia: Basic and Clinical Aspects. Curr Neuropharmacol 18:109–119. https://doi.org/10.2174/1570159x17666191010094350
Bendtsen L, Zakrzewska JM, Abbott J, Braschinsky M, Di Stefano G, Donnet A, Eide PK, Leal PRL, Maarbjerg S, May A, Nurmikko T, Obermann M, Jensen TS, Cruccu G (2019) European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol 26:831–849. https://doi.org/10.1111/ene.13950
Burchiel KJ (1980) Abnormal impulse generation in focally demyelinated trigeminal roots. J Neurosurg 53:674–683. https://doi.org/10.3171/jns.1980.53.5.0674
Cruccu G, Pennisi EM, Antonini G, Biasiotta A, di Stefano G, La Cesa S, Leone C, Raffa S, Sommer C, Truini A (2014) Trigeminal isolated sensory neuropathy (TISN) and FOSMN syndrome: despite a dissimilar disease course do they share common pathophysiological mechanisms? BMC Neurol 14:248. https://doi.org/10.1186/s12883-014-0248-2
Witty DR, Alvaro G, Derjean D, Giblin GMP, Gunn K, Large C, Macpherson DT, Morisset V, Owen D, Palmer J, Rugiero F, Tate S, Hinckley CA, Naik H (2020) Discovery of Vixotrigine: A Novel Use-Dependent Sodium Channel Blocker for the Treatment of Trigeminal Neuralgia. ACS Med Chem Lett 11:1678–1687. https://doi.org/10.1021/acsmedchemlett.0c00263
Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F (2015) Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag 11:289–299. https://doi.org/10.2147/tcrm.S37592
Célèrier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G (2000) Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology 92:465–472. https://doi.org/10.1097/00000542-200002000-00029
Chia YY, Liu K, Wang JJ, Kuo MC, Ho ST (1999) Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Can J Anaesth 46:872–877. https://doi.org/10.1007/bf03012978
Chen SR, Prunean A, Pan HM, Welker KL, Pan HL (2007) Resistance to morphine analgesic tolerance in rats with deleted transient receptor potential vanilloid type 1-expressing sensory neurons. Neuroscience 145:676–685. https://doi.org/10.1016/j.neuroscience.2006.12.016
Mao J, Price DD, Mayer DJ (1994) Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci 14:2301–2312. https://doi.org/10.1523/jneurosci.14-04-02301.1994
Osteen JD, Herzig V, Gilchrist J, Emrick JJ, Zhang C, Wang X, Castro J, Garcia-Caraballo S, Grundy L, Rychkov GY, Weyer AD, Dekan Z, Undheim EA, Alewood P, Stucky CL, Brierley SM, Basbaum AI, Bosmans F, King GF, Julius D (2016) Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 534:494–499. https://doi.org/10.1038/nature17976
Deuis JR, Zimmermann K, Romanovsky AA, Possani LD, Cabot PJ, Lewis RJ, Vetter I (2013) An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain 154:1749–1757. https://doi.org/10.1016/j.pain.2013.05.032
Li CL, Liu XF, Li GX, Ban MQ, Chen JZ, Cui Y, Zhang JH, Wu CF (2016) Antinociceptive Effects of AGAP, a Recombinant Neurotoxic Polypeptide: Possible Involvement of the Tetrodotoxin-Resistant Sodium Channels in Small Dorsal Root Ganglia Neurons. Front Pharmacol 7:496. https://doi.org/10.3389/fphar.2016.00496
Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC (1996) Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem 271:5953–5956. https://doi.org/10.1074/jbc.271.11.5953
Agarwal N, Offermanns S, Kuner R (2004) Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis 38:122–129. https://doi.org/10.1002/gene.20010
Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN (2003) The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 550:739–752. https://doi.org/10.1113/jphysiol.2003.042127
Padilla F, Couble ML, Coste B, Maingret F, Clerc N, Crest M, Ritter AM, Magloire H, Delmas P (2007) Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: implication for intestinal reflex function and orofacial pain. Mol Cell Neurosci 35:138–152. https://doi.org/10.1016/j.mcn.2007.02.008
Renganathan M, Cummins TR, Waxman SG (2001) Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86:629–640. https://doi.org/10.1152/jn.2001.86.2.629
Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN (2008) The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321:702–705. https://doi.org/10.1126/science.1156916
Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN (2003) GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol 548:373–382. https://doi.org/10.1113/jphysiol.2003.039131
Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ (2006) The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci 26:12852–12860. https://doi.org/10.1523/jneurosci.4015-06.2006
Leo S, D'Hooge R, Meert T (2010) Exploring the role of nociceptor-specific sodium channels in pain transmission using Nav1.8 and Nav1.9 knockout mice. Behav Brain Res 208:149–157. https://doi.org/10.1016/j.bbr.2009.11.023
Porreca F, Lai J, Bian D, Wegert S, Ossipov MH, Eglen RM, Kassotakis L, Novakovic S, Rabert DK, Sangameswaran L, Hunter JC (1999) A comparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc Natl Acad Sci U S A 96:7640–7644. https://doi.org/10.1073/pnas.96.14.7640
Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD (2008) Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci 28:3190–3201. https://doi.org/10.1523/jneurosci.4403-07.2008
Kawasaki Y, Zhang L, Cheng JK, Ji RR (2008) Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 28:5189–5194. https://doi.org/10.1523/jneurosci.3338-07.2008
Dworkin S, Heath JK, deJong-Curtain TA, Hogan BM, Lieschke GJ, Malaterre J, Ramsay RG, Mantamadiotis T (2007) CREB activity modulates neural cell proliferation, midbrain-hindbrain organization and patterning in zebrafish. Dev Biol 307:127–141. https://doi.org/10.1016/j.ydbio.2007.04.026
Wang X, Liu Y, Zhang H, Jin J, Ma Y, Leng Y (2021) Sinomenine alleviates dorsal root ganglia inflammation to inhibit neuropathic pain via the p38 MAPK/CREB signalling pathway. Eur J Pharmacol 897:173945. https://doi.org/10.1016/j.ejphar.2021.173945
Yu LN, Sun LH, Wang M, Wang LJ, Wu Y, Yu J, Wang WN, Zhang FJ, Li X, Yan M (2017) EphrinB-EphB Signaling Induces Hyperalgesia through ERK5/CREB Pathway in Rats. Pain Physician 20:E563–e574
Ruan JP, Mao QH, Lu WG, Cai XT, Chen J, Li Q, Fu Q, Yan HJ, Cao JL, Cao P (2018) Inhibition of spinal MAPKs by scorpion venom peptide BmK AGAP produces a sensory-specific analgesic effect. Mol Pain 14:1744806918761238. https://doi.org/10.1177/1744806918761238
Maatoug R, Jebali J, Guieu R, De Waard M, Kharrat R (2018) BotAF, a new Buthus occitanus tunetanus scorpion toxin, produces potent analgesia in rodents. Toxicon 149:72–85. https://doi.org/10.1016/j.toxicon.2018.01.003
Xu J, Jiang Y, Wan L, Wang Q, Huang Z, Liu Y, Wu Y, Chen Z, Liu X (2017) Feeding recombinant E. coli with GST-mBmKTX fusion protein increases the fecundity and lifespan of Caenorhabditis elegans. Peptides 89:1–8. https://doi.org/10.1016/j.peptides.2017.01.003
Webb B, Sali A (2016) Comparative Protein Structure Modeling Using MODELLER. Curr Protoc Bioinformatics 54:5.6.1–5.6.37. https://doi.org/10.1002/cpbi.3
Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM (1996) AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR 8:477–486. https://doi.org/10.1007/bf00228148
Hooft R, Vriend G, Sander C, Abola E (1996) Errors in protein structures. Nature 381:272–272
Rong C, Li L and Weng Z (2013) PROTEINS: Structure, Function, and Genetics 52:80–87 (2003) ZDOCK: An Initial-Stage Protein-Docking Algorithm.
Li L, Rong C, Weng Z (2003) RDOCK: refinement of Rigid-body Protein Docking Predictions. Proteins: Struct Funct Genet 53:693–707
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107. https://doi.org/10.1016/0304-3959(88)90209-6
Fu J, Mu G, Qiu L, Zhao J, Ou C (2020) c-Abl-p38alpha signaling pathway mediates dopamine neuron loss in trigeminal neuralgia. Mol Pain 16:1744806920930855. https://doi.org/10.1177/1744806920930855
Vos BP, Strassman AM, Maciewicz RJ (1994) Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J Neurosci 14:2708–2723. https://doi.org/10.1523/jneurosci.14-05-02708.1994
Pei L, Lin CY, Dai JP, Yin GF (2007) Facial pain induces the alteration of transient receptor potential vanilloid receptor 1 expression in rat trigeminal ganglion. Neurosci Bull 23:92–100. https://doi.org/10.1007/s12264-007-0013-2
Luo DS, Zhang T, Zuo CX, Zuo ZF, Li H, Wu SX, Wang W, Li YQ (2012) An animal model for trigeminal neuralgia by compression of the trigeminal nerve root. Pain Physician 15:187–196
von Hehn CA, Baron R, Woolf CJ (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73:638–652. https://doi.org/10.1016/j.neuron.2012.02.008
Ding W, You Z, Shen S, Yang J, Lim G, Doheny JT, Chen L, Zhu S, Mao J (2017) An Improved Rodent Model of Trigeminal Neuropathic Pain by Unilateral Chronic Constriction Injury of Distal Infraorbital Nerve. J Pain 18:899–907. https://doi.org/10.1016/j.jpain.2017.02.427
Rowe AH, Rowe MP (2008) Physiological resistance of grasshopper mice (Onychomys spp.) to Arizona bark scorpion (Centruroides exilicauda) venom. Toxicon 52:597–605. https://doi.org/10.1016/j.toxicon.2008.07.004
Rowe AH, Xiao Y, Rowe MP, Cummins TR, Zakon HH (2013) Voltage-gated sodium channel in grasshopper mice defends against bark scorpion toxin. Science 342:441–446. https://doi.org/10.1126/science.1236451
Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG (1999) A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci 19:Rc43. https://doi.org/10.1523/JNEUROSCI.19-24-j0001.1999
Decosterd I, Ji RR, Abdi S, Tate S, Woolf CJ (2002) The pattern of expression of the voltage-gated sodium channels Na(v)1.8 and Na(v)1.9 does not change in uninjured primary sensory neurons in experimental neuropathic pain models. Pain 96:269–277. https://doi.org/10.1016/s0304-3959(01)00456-0
Dib-Hajj S, Black JA, Felts P, Waxman SG (1996) Down-regulation of transcripts for Na channel alpha-SNS in spinal sensory neurons following axotomy. Proc Natl Acad Sci U S A 93:14950–14954. https://doi.org/10.1073/pnas.93.25.14950
Okuse K, Chaplan SR, McMahon SB, Luo ZD, Calcutt NA, Scott BP, Akopian AN, Wood JN (1997) Regulation of expression of the sensory neuron-specific sodium channel SNS in inflammatory and neuropathic pain. Mol Cell Neurosci 10:196–207. https://doi.org/10.1006/mcne.1997.0657
Sleeper AA, Cummins TR, Dib-Hajj SD, Hormuzdiar W, Tyrrell L, Waxman SG, Black JA (2000) Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy. J Neurosci 20:7279–7289. https://doi.org/10.1523/jneurosci.20-19-07279.2000
Sanna MD, Ghelardini C, Galeotti N (2016) Blockade of the spinal BDNF-activated JNK pathway prevents the development of antiretroviral-induced neuropathic pain. Neuropharmacology 105:543–552. https://doi.org/10.1016/j.neuropharm.2016.02.016
Kong F, Sun K, Zhu J, Li F, Lin F, Sun X, Luo X, Ren C, Lu L, Zhao S, Sun J, Wang Y, Shi J (2021) PD-L1 Improves Motor Function and Alleviates Neuropathic Pain in Male Mice After Spinal Cord Injury by Inhibiting MAPK Pathway. Front Immunol 12:670646. https://doi.org/10.3389/fimmu.2021.670646
Zhang T, Zhang R, Xu B, Zhang M, Zhang Q, Li N, Qiu Y, Chen D, Xu K, Xiao J, Zhang N, Fang Q (2021) Spinal endomorphins attenuate burn-injury pain in male mice by inhibiting p38 MAPK signaling pathway through the mu-opioid receptor. Eur J Pharmacol 903:174139. https://doi.org/10.1016/j.ejphar.2021.174139
Sundström E, Mo LL (2002) Mechanisms of glutamate release in the rat spinal cord slices during metabolic inhibition. J Neurotrauma 19:257–266. https://doi.org/10.1089/08977150252806992
Zahn PK, Sluka KA, Brennan TJ (2002) Excitatory amino acid release in the spinal cord caused by plantar incision in the rat. Pain 100:65–76. https://doi.org/10.1016/s0304-3959(02)00241-5
Lohr C, Deitmer JW (2006) Calcium signaling in invertebrate glial cells. Glia 54:642–649. https://doi.org/10.1002/glia.20368
Huang CT, Chen SH, Lin SC, Chen WT, Lue JH, Tsai YJ (2018) Erythropoietin reduces nerve demyelination, neuropathic pain behavior and microglial MAPKs activation through erythropoietin receptors on Schwann cells in a rat model of peripheral neuropathy. Glia 66:2299–2315. https://doi.org/10.1002/glia.23461
Liu X, Li C, Chen J, Du J, Zhang J, Li G, Jin X, Wu C (2014) AGAP, a new recombinant neurotoxic polypeptide, targets the voltage-gated calcium channels in rat small diameter DRG neurons. Biochem Biophys Res Commun 452:60–65. https://doi.org/10.1016/j.bbrc.2014.08.051
Cestèle S, Qu Y, Rogers JC, Rochat H, Scheuer T, Catterall WA (1998) Voltage sensor-trapping: enhanced activation of sodium channels by beta-scorpion toxin bound to the S3-S4 loop in domain II. Neuron 21:919–931. https://doi.org/10.1016/s0896-6273(00)80606-6
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant number: 81073081, 81973227), Scientific Research Foundation of the Education Department of Liaoning Province (2021), Scientific Research Staring Foundation for the Returned Overseas Scholars, Shenyang Pharmaceutical University (grant number: GGJJ2021101), and National Science and Technology Major Project of the Ministry of Science and Technology of China (grant number: 2018ZX09735005).
Funding
This research was funded by the National Natural Science Foundation of China (grant number: 81073081, 81973227), Scientific Research Foundation of the Education Department of Liaoning Province (2021), Scientific Research Staring Foundation for the Returned Overseas Scholars, Shenyang Pharmaceutical University (grant number: GGJJ2021101), and National Science and Technology Major Project of the Ministry of Science and Technology of China (grant number: 2018ZX09735005).
Author information
Authors and Affiliations
Contributions
Chun-li Li did conceptualization, supervision and funding acquisition; Hai-peng Wang, Chun-yun Chen and Bai Fei performed methodology; Ran Yang and Hai-peng Wang done formal analysis, visualization and data curation; Yong-bo Song did resources; Ran Yang contributed to writing—original draft preparation; Chun-li Li and Ran Yang performed writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Research Involving Human and Animal Participants
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Animal Ethics Committee of Shenyang Pharmaceutical University, China (SCXK (Liao) 2020-0001).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12035_2022_2855_MOESM1_ESM.docx
Supplementary file1 Supplementary Materials Fig. S1 (a1-f1) Homology modeling structure of BmK ANGP and its mutants. (a2-f2) Interaction model of BmK ANGP and its mutants with Nav1.8. Tab. S1. Results of acetic acid writhing test of BmK ANGP and its mutants. (DOCX 1417 kb)
Rights and permissions
About this article
Cite this article
Yang, R., Song, Y., Wang, H. et al. BmK DKK13, A Scorpion Toxin, Alleviates Pain Behavior in a Rat Model of Trigeminal Neuralgia by Modulating Voltage-Gated Sodium Channels and MAPKs/CREB Pathway. Mol Neurobiol 59, 4535–4549 (2022). https://doi.org/10.1007/s12035-022-02855-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02855-x