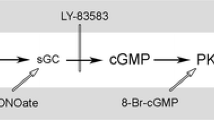
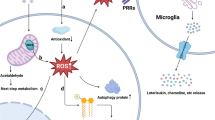
Abstract
Alcohol exposure during pregnancy is harmful to the fetus and causes a wide range of long-lasting physiological and neurocognitive impairments, collectively referred to as fetal alcohol spectrum disorders (FASD). The neurobehavioral deficits observed in FASD result from structural and functional damages in the brain, with neurodegeneration being the most destructive consequence. Currently, there are no therapies for FASD. It is exigent to delineate the underlying mechanisms of alcohol neurotoxicity and develop an effective strategy of treatment. ER stress, caused by the accumulation of unfolded/misfolded proteins in the ER, is the hallmark of many neurodegenerative diseases, including alcohol-induced neurodegeneration. Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a newly discovered endoplasmic reticulum (ER) stress responsive neurotrophic factor that regulates diverse neuronal functions. This review summarizes the recent findings revealing the effects of MANF on the CNS and its protective role against neurodegeneration. Particularly, we focus the role of MANF on alcohol-induced ER stress and neurodegeneration and discuss the therapeutic potential of MANF in treating alcohol neurotoxicity such as FASD.



Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Abbreviations
- FASD:
-
Fetal alcohol spectrum disorders
- FAS:
-
Fetal alcohol syndrome
- PAE:
-
Prenatal alcohol exposure
- ER:
-
Endoplasmic reticulum
- SER:
-
Smooth endoplasmic reticulum
- CNS:
-
Central nervous system
- MANF:
-
Mesencephalic astrocyte-derived neurotrophic factor
- CDNF:
-
Cerebral dopamine neurotrophic factor
- NTFs:
-
Neurotrophic factors
- BDNF:
-
Brain-derived neurotrophic factor
- GFLs:
-
Glial cell line-derived neurotrophic factor family of ligands
- GDNF:
-
Glial cell line-derived neurotrophic factor
- CNTF:
-
Ciliary neurotrophic factor
- IL-6:
-
Interleukin 6
- ROS:
-
Reactive oxygen species
- UPR:
-
Unfolded protein response
- PERK:
-
Pancreatic ER kinase-like ER kinase
- IRE1α:
-
Inositol-requiring enzyme 1 α
- ATF6:
-
Activating transcription factor 6
- XBP1s:
-
X-box binding protein 1
- GRP78:
-
Glucose-regulated protein 78 kDa
- ERAD:
-
ER-associated protein degradation
- PD:
-
Parkinson’s disease
- AD:
-
Alzheimer’s disease
- ARP:
-
Arginine-rich protein
- UTR:
-
Untranslated region
- SAPLIP:
-
Saposin-like protein
- PDIs:
-
Protein disulfide isomerases
- SCG:
-
Superior cervical ganglion
- KDELRs:
-
KDEL receptors
- TH:
-
Tyrosine hydroxylase
- KO:
-
Knock-out
- NSCs:
-
Neural stem cells
- MCAO:
-
Middle cerebral artery occlusion
- ERSE:
-
ER stress response element
- AAV:
-
Adeno-associated virus
- BMSC:
-
Bone marrow mesenchymal stem cells
- 6-OHDA:
-
6-Hydroxydopamine
- HSP70:
-
Heat shock protein 70
- Aβ:
-
Amyloid β-peptide
- APP/PS1:
-
Amyloid precursor protein/presenilin 1
- ITGC:
-
Inferior temporal gyrus of the cortex
- SCA17:
-
Spinocerebellar ataxia 17
- TBP:
-
TATA box binding protein
- RP:
-
Retinitis pigmentosa
- sI/R:
-
Simulated ischemia/reperfusion
- MRI:
-
Magnetic resonance imaging
- GD:
-
Gestation days
- PD:
-
Postnatal days
- ADH:
-
Alcohol dehydrogenase
- CYP2E1:
-
Cytochrome P450 2E1
- ALDH:
-
Aldehyde dehydrogenase
- BAC:
-
Blood alcohol concentration
- BBB:
-
Blood-brain barrier
- 4-PBA:
-
4-Phenylbutyric acid
- MEFs:
-
Mouse embryonic fibroblasts
- Bcl2:
-
Bcl2 apoptosis regulator
- Bcl2l1:
-
Bcl2 like 1
- Bax:
-
Bcl2 associated X apoptosis regulator
- Bak1:
-
Bcl2 antagonist/killer 1
- Bad:
-
Bcl2 associated agonist of cell death
- Bid:
-
BH3 interacting domain death agonist
- Bnip3:
-
Bcl2 interacting protein 3
- cHAP:
-
Crossed high alcohol preferring
- SERCA:
-
Sarco/endoplasmic reticulum Ca2+
- ATP:
-
Adenosine tri-phosphate
- ADP:
-
Adenosine di-phosphate
References
Voutilainen MH et al (2015) Therapeutic potential of the endoplasmic reticulum located and secreted CDNF/MANF family of neurotrophic factors in Parkinson’s disease. FEBS Lett 589(24 Pt A):3739–3748
Cui Q (2006) Actions of neurotrophic factors and their signaling pathways in neuronal survival and axonal regeneration. Mol Neurobiol 33(2):155–179
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Petrova P et al (2003) MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 20(2):173–188
Lindholm P et al (2007) Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 448(7149):73–77
Lindahl M, Saarma M, Lindholm P (2017) Unconventional neurotrophic factors CDNF and MANF: structure, physiological functions and therapeutic potential. Neurobiol Dis 97(Pt B):90–102
Glembotski CC et al (2012) Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem 287(31):25893–25904
Mizobuchi N et al (2007) ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct 32(1):41–50
Oh-Hashi K et al (2012) Intracellular trafficking and secretion of mouse mesencephalic astrocyte-derived neurotrophic factor. Mol Cell Biochem 363(1-2):35–41
Voutilainen MH et al (2009) Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci 29(30):9651–9659
Airavaara M et al (2009) Mesencephalic astrocyte-derived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J Comp Neurol 515(1):116–124
Xu S et al (2019) Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Abeta toxicity via attenuating Abeta-induced endoplasmic reticulum stress. J Neuroinflammation 16(1):35
Hellman M et al (2011) Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J Biol Chem 286(4):2675–2680
Lindholm P et al (2008) MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol Cell Neurosci 39(3):356–371
Wang H et al (2014) Spatiotemporal expression of MANF in the developing rat brain. PLoS One 9(2):e90433
Danilova T et al (2019) Mesencephalic astrocyte-derived neurotrophic factor (MANF) is highly expressed in mouse tissues with metabolic function. Front Endocrinol (Lausanne) 10:765
Chen YC et al (2012) MANF regulates dopaminergic neuron development in larval zebrafish. Dev Biol 370(2):237–249
Palgi M et al (2009) Evidence that DmMANF is an invertebrate neurotrophic factor supporting dopaminergic neurons. Proc Natl Acad Sci U S A 106(7):2429–2434
Wen W et al (2020) Mesencephalic astrocyte-derived neurotrophic factor (MANF) regulates neurite outgrowth through the activation of Akt/mTOR and Erk/mTOR signaling pathways. Front Mol Neurosci 13(188)
Tseng K-Y et al (2017) MANF is essential for neurite extension and neuronal migration in the developing cortex. eneuro 4(5)
SAMHSA, 2019 National Survey on Drug Use and Health (NSDUH). Retrieved from https://www.samhsa.gov/data/report/2019-nsduh-annual-national-report, 2019.
Muggli E et al (2016) “Did you ever drink more?” A detailed description of pregnant women’s drinking patterns. BMC Public Health 16:683
Nykjaer C et al (2014) Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: evidence from a British cohort. J Epidemiol Community Health 68(6):542–549
Dejong K, Olyaei A, Lo JO (2019) Alcohol use in pregnancy. Clin Obstet Gynecol 62(1):142–155
Tan CH et al (2015) Alcohol use and binge drinking among women of childbearing age - United States, 2011–2013. MMWR Morb Mortal Wkly Rep 64(37):1042–1046
CDC (2012) Alcohol use and binge drinking among women of childbearing age—United States, 2006–2010. MMWR Morb Mortal Wkly Rep 61(28):534–538
Denny CH et al (2019) Consumption of alcohol beverages and binge drinking among pregnant women aged 18–44 years - United States, 2015–2017. MMWR Morb Mortal Wkly Rep 68(16):365–368
Ethen MK et al (2009) Alcohol consumption by women before and during pregnancy. Matern Child Health J 13(2):274–285
Crews FT, Nixon K (2009) Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 44(2):115–127
May PA et al (2014) Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 134(5):855–866
Wilhoit LF, Scott DA, Simecka BA (2017) Fetal alcohol spectrum disorders: characteristics, complications, and treatment. Community Ment Health J 53(6):711–718
Hoyme HE et al (2016) Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 138(2)
Luo J (2009) GSK3beta in ethanol neurotoxicity. Mol Neurobiol 40(2):108–121
Mooney S, Lein P, Miller M (2013) Comprehensive developmental neuroscience: neural circuit development and function in the heathy and diseased brain: chapter 28. Fetal alcohol spectrum disorder: targeted effects of ethanol on cell proliferation and survival. Elsevier Inc. Chapters
Crews FT, Vetreno RP (2016) Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology 233(9):1543–1557
Chastain LG, Sarkar DK (2014) Role of microglia in regulation of ethanol neurotoxic action. Int Rev Neurobiol 118:81–103
Haorah J et al (2008) Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med 45(11):1542–1550
Hernández JA, López-Sánchez RC, Rendón-Ramírez A (2016) Lipids and oxidative stress associated with ethanol-induced neurological damage. Oxidative Med Cell Longev 2016:1543809
Buján GE et al (2019) Oxidative stress-induced brain damage triggered by voluntary ethanol consumption during adolescence: a potential target for neuroprotection? Curr Pharm Des 25(45):4782–4790
Maier SE, Chen WJ, West JR (1996) Prenatal binge-like alcohol exposure alters neurochemical profiles in fetal rat brain. Pharmacol Biochem Behav 55(4):521–529
Sanderson JL, Donald Partridge L, Valenzuela CF (2009) Modulation of GABAergic and glutamatergic transmission by ethanol in the developing neocortex: an in vitro test of the excessive inhibition hypothesis of fetal alcohol spectrum disorder. Neuropharmacology 56(2):541–555
Luo J, Miller MW (1998) Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev 27(2):157–167
Ke Z et al (2011) Ethanol induces endoplasmic reticulum stress in the developing brain. Alcohol Clin Exp Res 35(9):1574–1583
Li H et al (2019) 4-Phenylbutyric acid protects against ethanol-induced damage in the developing mouse brain. Alcohol Clin Exp Res 43(1):69–78
Alimov A et al (2013) Expression of autophagy and UPR genes in the developing brain during ethanol-sensitive and resistant periods. Metab Brain Dis 28(4):667–676
Yang F, Luo J (2015) Endoplasmic reticulum stress and ethanol neurotoxicity. Biomolecules 5(4):2538–2553
Wang Y et al (2018) Binge ethanol exposure induces endoplasmic reticulum stress in the brain of adult mice. Toxicol Appl Pharmacol 356:172–181
Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13(8):477–491
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13(3):385–392
Xin Q et al (2014) Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int 68:18–27
Liu S et al (2015) Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis 6(1):e1582
Andhavarapu S et al (2019) Interplay between ER stress and autophagy: a possible mechanism in multiple sclerosis pathology. Exp Mol Pathol 108:183–190
O'Brien PD et al (2014) ER stress in diabetic peripheral neuropathy: a new therapeutic target. Antioxid Redox Signal 21(4):621–633
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086
Rasheva VI, Domingos PM (2009) Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14(8):996–1007
Apostolou A et al (2008) Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp Cell Res 314(13):2454–2467
Jӓntti M, Harvey BK (2020) Trophic activities of endoplasmic reticulum proteins CDNF and MANF. Cell Tissue Res
Tadimalla A et al (2008) Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res 103(11):1249–1258
Li-Na Z et al (2017) Mesencephalic astrocyte-derived neurotrophic factor and its role in nervous system disease. Neurol Sci
Wang Y et al (2020) MANF is neuroprotective against ethanol-induced neurodegeneration through ameliorating ER stress. Neurobiol Dis:105216
Shridhar R et al (1996) Mutations in the arginine-rich protein gene, in lung, breast, and prostate cancers, and in squamous cell carcinoma of the head and neck. Cancer Res 56(24):5576–5578
Evron E et al (1997) Normal polymorphism in the incomplete trinucleotide repeat of the arginine-rich protein gene. Cancer Res 57(14):2888–2889
Hellman M et al (2010) 1H, 13C and 15N resonance assignments of the human mesencephalic astrocyte-derived neurotrophic factor. Biomol NMR Assign 4(2):215–217
Hoseki J et al (2010) Solution structure and dynamics of mouse ARMET. FEBS Lett 584(8):1536–1542
Parkash V et al (2009) The structure of the conserved neurotrophic factors MANF and CDNF explains why they are bifunctional. Protein Eng Des Sel 22(4):233–241
Bai M et al (2018) Conserved roles of C. elegans and human MANFs in sulfatide binding and cytoprotection. Nat Commun 9(1):897
Sereno D et al (2017) An evolutionary perspective on the role of mesencephalic astrocyte-derived neurotrophic factor (MANF): at the crossroads of poriferan innate immune and apoptotic pathways. Biochem Biophys Rep 11:161–173
Wang W et al (2015) Armet is an effector protein mediating aphid-plant interactions. FASEB J 29(5):2032–2045
Lindholm P, Saarma M (2010) Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol 70(5):360–371
Lindstrom R et al (2013) Characterization of the structural and functional determinants of MANF/CDNF in Drosophila in vivo model. PLoS One 8(9):e73928
Lindahl M et al (2014) MANF is indispensable for the proliferation and survival of pancreatic beta cells. Cell Rep 7(2):366–375
Chen L et al (2015) Mesencephalic astrocyte-derived neurotrophic factor is involved in inflammation by negatively regulating the NF-kappaB pathway. Sci Rep 5:8133
Hakonen E et al (2018) MANF protects human pancreatic beta cells against stress-induced cell death. Diabetologia
Yan Y et al (2019) MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP. Nat Commun 10(1):541
Matlik K et al (2015) Role of two sequence motifs of mesencephalic astrocyte-derived neurotrophic factor in its survival-promoting activity. Cell Death Dis 6:e2032
Bozok V et al (2018) Antioxidative CXXC peptide motif from mesencephalic astrocyte-derived neurotrophic factor antagonizes programmed cell death. Front Cell Dev Biol 6:106
Henderson MJ et al (2013) Mesencephalic astrocyte-derived neurotrophic factor (MANF) secretion and cell surface binding are modulated by KDEL receptors. J Biol Chem 288(6):4209–4225
Jia J et al (2020) KDEL receptor is a cell surface receptor that cycles between the plasma membrane and the Golgi via clathrin-mediated transport carriers. Cell Mol Life Sci
Vaccaro AM et al (1999) Saposins and their interaction with lipids. Neurochem Res 24(2):307–314
Hada M et al (2016) Cytosolic Ku70 regulates Bax-mediated cell death. Tumour Biol 37(10):13903–13914
Quan S et al (2007) The CXXC motif is more than a redox rheostat. J Biol Chem 282(39):28823–28833
Yagi T et al (2020) Neuroplastin modulates anti-inflammatory effects of MANF. iScience 23(12):101810
Kovaleva, V., et al., MANF regulates unfolded protein response and neuronal survival through its ER-located receptor IRE1α. bioRxiv, 2020: p. 2020.09.22.307744.
Yang S et al (2017) MANF regulates hypothalamic control of food intake and body weight. Nat Commun 8(1):579
Liu J et al (2015) ER stress-inducible protein MANF selectively expresses in human spleen. Hum Immunol 76(11):823–830
Stratoulias V, Heino TI (2015) Analysis of the conserved neurotrophic factor MANF in the Drosophila adult brain. Gene Expr Patterns 18(1-2):8–15
Yavarna T et al (2015) High diagnostic yield of clinical exome sequencing in Middle Eastern patients with Mendelian disorders. Hum Genet 134(9):967–980
Montaser H et al (2021) Loss of MANF causes childhood onset syndromic diabetes due to increased endoplasmic reticulum stress. Diabetes
Pakarinen E et al (2020) MANF ablation causes prolonged activation of the UPR without neurodegeneration in the mouse midbrain dopamine system. eNeuro
Richman C et al (2018) C. elegans MANF homolog is necessary for the protection of dopaminergic neurons and ER unfolded protein response. Front Neurosci 12:544
Wang Y, Wen W, Li H, Xu H, Xu M, Ma M, Luo J (2022) Deficiency of Mesencephalic Astrocyte-derived Neurotrophic Factor Affects Neurogenesis in Mouse Brain. Brain Res Bull S0361–9230(22):00058–00052. https://doi.org/10.1016/j.brainresbull.2022.02.019
Airavaara M et al (2010) Widespread cortical expression of MANF by AAV serotype 7: localization and protection against ischemic brain injury. Exp Neurol 225(1):104–113
Yu YQ et al (2010) Induction profile of MANF/ARMET by cerebral ischemia and its implication for neuron protection. J Cereb Blood Flow Metab 30(1):79–91
Shen Y et al (2012) Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation. J Neuroinflammation 9:254
Wang H et al (2015) Tunicamycin-induced unfolded protein response in the developing mouse brain. Toxicol Appl Pharmacol 283(3):157–167
Yoshida H et al (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273(50):33741–33749
Yoshida H et al (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20(18):6755–6767
Kokame K, Kato H, Miyata T (2001) Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J Biol Chem 276(12):9199–9205
Oh-Hashi K, Hirata Y, Kiuchi K (2013) Transcriptional regulation of mouse mesencephalic astrocyte-derived neurotrophic factor in Neuro2a cells. Cell Mol Biol Lett 18(3):398–415
Yamamoto K et al (2004) Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem 136(3):343–350
Wang D et al (2018) XBP1 activation enhances MANF expression via binding to endoplasmic reticulum stress response elements within MANF promoter region in hepatitis B. Int J Biochem Cell Biol 99:140–146
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23(21):7448–7459
Yang S et al (2014) Age-dependent decrease in chaperone activity impairs MANF expression, leading to Purkinje cell degeneration in inducible SCA17 mice. Neuron 81(2):349–365
Martindale JJ et al (2006) Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res 98(9):1186–1193
Kim Y et al (2016) Mesencephalic astrocyte-derived neurotrophic factor as a urine biomarker for endoplasmic reticulum stress-related kidney diseases. J Am Soc Nephrol 27(10):2974–2982
Tousson-Abouelazm N et al (2020) Urinary ERdj3 and mesencephalic astrocyte-derived neutrophic factor identify endoplasmic reticulum stress in glomerular disease. Lab Investig 100(7):945–958
Fu J et al (2020) Liraglutide protects pancreatic β cells from endoplasmic reticulum stress by upregulating MANF to promote autophagy turnover. Life Sci 252:117648
Galli E et al (2016) Increased circulating concentrations of mesencephalic astrocyte-derived neurotrophic factor in children with type 1 diabetes. Sci Rep 6:29058
Llewellyn DH, Roderick HL, Rose S (1997) KDEL receptor expression is not coordinatedly up-regulated with ER stress-induced reticuloplasmin expression in HeLa cells. Biochem Biophys Res Commun 240(1):36–40
Glembotski CC (2011) Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J Mol Cell Cardiol 51(4):512–517
Eesmaa A et al (2021) The cytoprotective protein MANF promotes neuronal survival independently from its role as a GRP78 cofactor. J Biol Chem:100295
Lindstrom R et al (2016) Exploring the conserved role of MANF in the unfolded protein response in Drosophila melanogaster. PLoS One 11(3):e0151550
Huang J et al (2016) Mesencephalic astrocyte-derived neurotrophic factor reduces cell apoptosis via upregulating GRP78 in SH-SY5Y cells. Cell Biol Int 40(7):803–811
Hartman JH et al (2019) MANF deletion abrogates early larval Caenorhabditis elegans stress response to tunicamycin and Pseudomonas aeruginosa. Eur J Cell Biol
Herranen A et al (2020) Deficiency of the ER-stress-regulator MANF triggers progressive outer hair cell death and hearing loss. Cell Death Dis 11(2):100
Doyle KM et al (2011) Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med 15(10):2025–2039
Yang W et al (2014) Mesencephalic astrocyte-derived neurotrophic factor prevents neuron loss via inhibiting ischemia-induced apoptosis. J Neurol Sci 344(1-2):129–138
Wang XY et al (2016) MRI dynamically evaluates the therapeutic effect of recombinant human MANF on ischemia/reperfusion injury in rats. Int J Mol Sci 17(9)
Matlik K et al (2018) Poststroke delivery of MANF promotes functional recovery in rats. Sci Adv 4(5):eaap8957
Teppo J et al (2020) Molecular profile of the rat peri-infarct region four days after stroke: study with MANF. Exp Neurol 329:113288
Gao B et al (2020) Effects of mesencephalic astrocyte-derived neurotrophic factor on cerebral angiogenesis in a rat model of cerebral ischemia. Neurosci Lett 715:134657
Yang F et al (2020) Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling. World J Stem Cells 12(7):633–658
Belayev L et al (2020) DHA modulates MANF and TREM2 abundance, enhances neurogenesis, reduces infarct size, and improves neurological function after experimental ischemic stroke. CNS Neurosci Ther 26(11):1155–1167
Cordero-Llana O et al (2015) Enhanced efficacy of the CDNF/MANF family by combined intranigral overexpression in the 6-OHDA rat model of Parkinson’s disease. Mol Ther 23(2):244–254
Hao F et al (2017) Long-term protective effects of AAV9-mesencephalic astrocyte-derived neurotrophic factor gene transfer in Parkinsonian rats. Exp Neurol
Sun H et al (2017) Mesencephalic astrocyte-derived neurotrophic factor reduces cell apoptosis via upregulating HSP70 in SHSY-5Y cells. Transl Neurodegener 6:12
Zhang J et al (2017) Mesencephalic astrocyte-derived neurotrophic factor alleviated 6-OHDA-induced cell damage via ROS-AMPK/mTOR mediated autophagic inhibition. Exp Gerontol 89:45–56
Zhang J et al (2017) Nrf2-mediated neuroprotection by MANF against 6-OHDA-induced cell damage via PI3K/AKT/GSK3beta pathway. Exp Gerontol 100:77–86
Liu Y et al (2018) MANF improves the MPP(+)/MPTP-induced Parkinson’s disease via improvement of mitochondrial function and inhibition of oxidative stress. Am J Transl Res 10(5):1284–1294
Zhang Z et al (2018) MANF protects dopamine neurons and locomotion defects from a human alpha-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp Neurol
Galli E et al (2019) Increased serum levels of mesencephalic astrocyte-derived neurotrophic factor in subjects with Parkinson’s disease. Front Neurosci 13:929
Virachit S et al (2019) Levels of glial cell line-derived neurotrophic factor are decreased, but fibroblast growth factor 2 and cerebral dopamine neurotrophic factor are increased in the hippocampus in Parkinson’s disease. Brain Pathol 29(6):813–825
Liu X-C et al (2021) Increased MANF expression in the inferior temporal gyrus in patients with Alzheimer disease. Frontiers in Aging. Neuroscience 13(220)
Guo J et al (2018) Piperine ameliorates SCA17 neuropathology by reducing ER stress. Mol Neurodegener 13(1):4
Neves J et al (2016) Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science 353(6294):aaf3646
Gao FJ et al (2017) Identification of mesencephalic astrocyte-derived neurotrophic factor as a novel neuroprotective factor for retinal ganglion cells. Front Mol Neurosci 10:76
Lu J et al (2018) Photoreceptor protection by mesencephalic astrocyte-derived neurotrophic factor (MANF). eNeuro 5(2)
McLaughlin T et al (2018) p58(IPK) is an endogenous neuroprotectant for retinal ganglion cells. Front Aging Neurosci 10:267
Wang X et al (2020) MANF promotes diabetic corneal epithelial wound healing and nerve regeneration by attenuating hyperglycemia-induced endoplasmic reticulum stress. Diabetes 69(6):1264–1278
Neves J et al (2020) MANF delivery improves retinal homeostasis and cell replacement therapies in ageing mice. Exp Gerontol 134:110893
DeGracia DJ, Montie HL (2004) Cerebral ischemia and the unfolded protein response. J Neurochem 91(1):1–8
Tseng KY et al (2017) MANF promotes differentiation and migration of neural progenitor cells with potential neural regenerative effects in stroke. Mol Ther
Ryu EJ et al (2002) Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci 22(24):10690–10698
Mercado G et al (2016) ER stress and Parkinson’s disease: pathological inputs that converge into the secretory pathway. Brain Res 1648(Pt B):626–632
Yang C, Gao Y (2020) Mesencephalic astrocyte-derived neurotrophic factor: a treatment option for Parkinson’s disease. Front Biosci (Landmark Ed) 25:1718–1731
Albert K, Airavaara M (2019) Neuroprotective and reparative effects of endoplasmic reticulum luminal proteins - mesencephalic astrocyte-derived neurotrophic factor and cerebral dopamine neurotrophic factor. Croat Med J 60(2):99–108
De Strooper B, Karran E (2016) The cellular phase of Alzheimer’s disease. Cell 164(4):603–615
Huang HC et al (2015) Endoplasmic reticulum stress as a novel neuronal mediator in Alzheimer’s disease. Neurol Res 37(4):366–374
Gerakis Y, Hetz C (2018) Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J 285(6):995–1011
Kurt MA et al (2001) Neurodegenerative changes associated with beta-amyloid deposition in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Exp Neurol 171(1):59–71
Walkowicz L et al (2017) Downregulation of DmMANF in glial cells results in neurodegeneration and affects sleep and lifespan in Drosophila melanogaster. Front Neurosci 11:610
Gao FJ et al (2017) Expression and distribution of mesencephalic astrocyte-derived neurotrophic factor in the retina and optic nerve. Front Hum Neurosci 10:686
Sousa-Victor P, Jasper H, Neves J (2018) Trophic factors in inflammation and regeneration: the role of MANF and CDNF. Front Physiol 9:1629
Lemoine, P., H. Harrousseau, and J. Borteyru, Les enfants de parents alcooliques. Anomalies observees: a propos de 127 cas. 4Jd, 1968. 25 p. 476.
Jones KL, Smith DW (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet 302(7836):999–1001
Jones KL (2011) The effects of alcohol on fetal development. Birth Defects Res C Embryo Today 93(1):3–11
Jarmasz JS et al (2017) Human brain abnormalities associated with prenatal alcohol exposure and fetal alcohol spectrum disorder. J Neuropathol Exp Neurol 76(9):813–833
Boschen KE, Klintsova AY (2017) Neurotrophins in the brain: interaction with alcohol exposure during development. Vitam Horm 104:197–242
Caputo C, Wood E, Jabbour L (2016) Impact of fetal alcohol exposure on body systems: a systematic review. Birth Defects Res C Embryo Today 108(2):174–180
Petrelli B, Weinberg J, Hicks GG (2018) Effects of prenatal alcohol exposure (PAE): insights into FASD using mouse models of PAE. Biochem Cell Biol 96(2):131–147
Jones KL, Smith DW (1975) The fetal alcohol syndrome. Teratology 12(1):1–10
Clarren SK et al (1978) Brain malformations related to prenatal exposure to ethanol. J Pediatr 92(1):64–67
Wisniewski K et al (1983) A clinical neuropathological study of the fetal alcohol syndrome. Neuropediatrics 14(4):197–201
Lebel C, Roussotte F, Sowell ER (2011) Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev 21(2):102–118
Donald KA et al (2015) Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatr 27(5):251–269
Jacobson SW et al (2017) Heavy prenatal alcohol exposure is related to smaller corpus callosum in newborn MRI scans. Alcohol Clin Exp Res 41(5):965–975
Sowell ER et al (2008) Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex 18(1):136–144
Sowell ER et al (2002) Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage 17(4):1807–1819
Sowell ER et al (2008) Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci 28(6):1313–1319
Bookstein FL et al (2002) Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec 269(3):162–174
Wozniak JR et al (2009) Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res 33(10):1825–1835
O'Hare ED et al (2005) Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport 16(12):1285–1290
Sowell ER et al (1996) Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I-V. Alcohol Clin Exp Res 20(1):31–34
Autti-Rämö I et al (2002) MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol 44(2):98–106
Mattson SN, Bernes GA, Doyle LR (2019) Fetal alcohol spectrum disorders: a review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol Clin Exp Res 43(6):1046–1062
Nunez CC, Roussotte F, Sowell ER (2011) Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol Res Health 34(1):121–131
Tsang TW et al (2016) Prenatal alcohol exposure, FASD, and child behavior: a meta-analysis. Pediatrics 137(3):e20152542
Roussotte FF et al (2012) Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp 33(4):920–937
Hendrickson TJ et al (2017) Cortical gyrification is abnormal in children with prenatal alcohol exposure. Neuroimage Clin 15:391–400
Schmahmann JD (2019) The cerebellum and cognition. Neurosci Lett 688:62–75
Sullivan EV et al (1995) Alcohol and the cerebellum: effects on balance, motor coordination, and cognition. Alcohol Health Res World 19(2):138–141
Roebuck TM et al (1998) Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res 22(1):252–258
Valenzuela CF, Lindquist B, Zamudio-Bulcock PA (2010) A review of synaptic plasticity at Purkinje neurons with a focus on ethanol-induced cerebellar dysfunction. Int Rev Neurobiol 91:339–372
Sowell ER et al (2001) Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology 57(2):235–244
Biffen SC et al (2017) Reductions in corpus callosum volume partially mediate effects of prenatal alcohol exposure on IQ. Front Neuroanat 11:132
Ma X et al (2005) Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res 29(7):1214–1222
Paolozza A et al (2014) Response inhibition deficits in children with fetal alcohol spectrum disorder: relationship between diffusion tensor imaging of the corpus callosum and eye movement control. Neuroimage Clin 5:53–61
Almeida L et al (2020) Murine models for the study of fetal alcohol spectrum disorders: an overview. Front Pediatr 8:359
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(Suppl 3):511–533
Dobbing J, Sands J (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3(1):79–83
Khan I, Leventhal BL Developmental delay, in StatPearls. 2021, StatPearls Publishing Copyright © 2021. StatPearls Publishing LLC, Treasure Island
Koop M et al (1986) Volumetric development of the fetal telencephalon, cerebral cortex, diencephalon, and rhombencephalon including the cerebellum in man. Bibl Anat 28:53–78
Chen VS et al (2017) Histology atlas of the developing prenatal and postnatal mouse central nervous system, with emphasis on prenatal days E7.5 to E18.5. Toxicol Pathol 45(6):705–744
Gil-Mohapel J et al (2010) Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev 64(2):283–303
Parnell SE et al (2014) Dysmorphogenic effects of first trimester-equivalent ethanol exposure in mice: a magnetic resonance microscopy-based study. Alcohol Clin Exp Res 38(7):2008–2014
Fish EW et al (2016) Acute alcohol exposure during neurulation: behavioral and brain structural consequences in adolescent C57BL/6J mice. Behav Brain Res 311:70–80
Sulik KK, Johnston MC (1983) Sequence of developmental alterations following acute ethanol exposure in mice: craniofacial features of the fetal alcohol syndrome. Am J Anat 166(3):257–269
Dunty WC Jr et al (2001) Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res 25(10):1523–1535
Takahashi T et al (1999) Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci 19(23):10357–10371
Cui ZJ et al (2010) Prenatal alcohol exposure induces long-term changes in dendritic spines and synapses in the mouse visual cortex. Alcohol Alcohol 45(4):312–319
El Shawa H, Abbott CW 3rd, Huffman KJ (2013) Prenatal ethanol exposure disrupts intraneocortical circuitry, cortical gene expression, and behavior in a mouse model of FASD. J Neurosci 33(48):18893–18905
Barnes DE, Walker DW (1981) Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Brain Res 227(3):333–340
Miller MW (1996) Limited ethanol exposure selectively alters the proliferation of precursor cells in the cerebral cortex. Alcohol Clin Exp Res 20(1):139–143
Kotkoskie LA, Norton S (1989) Morphometric analysis of developing rat cerebral cortex following acute prenatal ethanol exposure. Exp Neurol 106(3):283–288
Miranda RC et al (2008) Modeling the impact of alcohol on cortical development in a dish: strategies from mapping neural stem cell fate. Methods Mol Biol 447:151–168
Xu W et al (2020) Early ethanol exposure inhibits the differentiation of hippocampal dentate gyrus granule cells in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 44(5):1112–1122
Ikonomidou C et al (2000) Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 287(5455):1056–1060
Gursky ZH, Spillman EC, Klintsova AY (2020) Single-day postnatal alcohol exposure induces apoptotic cell death and causes long-term neuron loss in rodent thalamic nucleus reuniens. Neuroscience 435:124–134
Idrus NM, Napper RM (2012) Acute and long-term Purkinje cell loss following a single ethanol binge during the early third trimester equivalent in the rat. Alcohol Clin Exp Res 36(8):1365–1373
Olney JW et al (2002) Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis 9(2):205–219
Olney JW et al (2002) Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res 133(2):115–126
Goodlett CR, Marcussen BL, West JR (1990) A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol 7(2):107–114
Kleiber ML et al (2014) Third trimester-equivalent ethanol exposure is characterized by an acute cellular stress response and an ontogenetic disruption of genes critical for synaptic establishment and function in mice. Dev Neurosci 36(6):499–519
Saito M et al (2016) Ethanol-induced neurodegeneration and glial activation in the developing brain. Brain Sci 6(3)
Goodlett CR et al (1993) Transient cortical astrogliosis induced by alcohol exposure during the neonatal brain growth spurt in rats. Brain Res Dev Brain Res 72(1):85–97
Lowery RL et al (2021) Microglia and astrocytes show limited, acute alterations in morphology and protein expression following a single developmental alcohol exposure. J Neurosci Res
Farber NB, Creeley CE, Olney JW (2010) Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol Dis 40(1):200–206
Creeley CE, Olney JW (2013) Drug-induced apoptosis: mechanism by which alcohol and many other drugs can disrupt brain development. Brain Sci 3(3):1153–1181
Marquardt K, Brigman JL (2016) The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: insights from rodent models. Alcohol 51:1–15
Cantacorps L et al (2018) Altered brain functional connectivity and behaviour in a mouse model of maternal alcohol binge-drinking. Prog Neuro-Psychopharmacol Biol Psychiatry 84(Pt A):237–249
Wozniak DF et al (2004) Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis 17(3):403–414
Hunt WA (1996) Role of acetaldehyde in the actions of ethanol on the brain—a review. Alcohol 13(2):147–151
Quertemont E et al (2005) The role of acetaldehyde in the central effects of ethanol. Alcohol Clin Exp Res 29(2):221–234
Zakhari S (2006) Overview: how is alcohol metabolized by the body? Alcohol Res Health 29(4):245–254
Hempel J et al (1984) Human liver alcohol dehydrogenase. 1. The primary structure of the beta 1 beta 1 isoenzyme. Eur J Biochem 145(3):437–445
Edenberg HJ (2007) The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health 30(1):5–13
Lieber CS, DeCarli LM (1972) The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J Pharmacol Exp Ther 181(2):279–287
Aragon CM, Spivak K, Amit Z (1985) Blockade of ethanol induced conditioned taste aversion by 3-amino-1,2,4-triazole: evidence for catalase mediated synthesis of acetaldehyde in rat brain. Life Sci 37(22):2077–2084
Weiner H, Wang X (1994) Aldehyde dehydrogenase and acetaldehyde metabolism. Alcohol Alcohol Suppl 2:141–145
Aragon CM, Rogan F, Amit Z (1992) Ethanol metabolism in rat brain homogenates by a catalase-H2O2 system. Biochem Pharmacol 44(1):93–98
Zimatkin SM, Buben AL (2007) Ethanol oxidation in the living brain. Alcohol Alcohol 42(6):529–532
Galter D et al (2003) Distribution of class I, III and IV alcohol dehydrogenase mRNAs in the adult rat, mouse and human brain. Eur J Biochem 270(6):1316–1326
Beisswenger TB, Holmquist B, Vallee BL (1985) chi-ADH is the sole alcohol dehydrogenase isozyme of mammalian brains: implications and inferences. Proc Natl Acad Sci U S A 82(24):8369–8373
Martínez SE et al (2001) Distribution of alcohol dehydrogenase mRNA in the rat central nervous system. Consequences for brain ethanol and retinoid metabolism. Eur J Biochem 268(19):5045–5056
Zimatkin SM, Lindros KO (1996) Distribution of catalase in rat brain: aminergic neurons as possible targets for ethanol effects. Alcohol Alcohol 31(2):167–174
Hipólito L et al (2007) Brain metabolism of ethanol and alcoholism: an update. Curr Drug Metab 8(7):716–727
Cohen G, Sinet PM, Heikkila R (1980) Ethanol oxidation by rat brain in vivo. Alcohol Clin Exp Res 4(4):366–370
Peana AT et al (2015) Role of ethanol-derived acetaldehyde in operant oral self-administration of ethanol in rats. Psychopharmacology 232(23):4269–4276
Sohda T et al (1993) Immunohistochemical demonstration of ethanol-inducible P450 2E1 in rat brain. Alcohol Alcohol Suppl 1b:69–75
Upadhya SC et al (2000) Cytochrome P4502E (CYP2E) in brain: constitutive expression, induction by ethanol and localization by fluorescence in situ hybridization. Arch Biochem Biophys 373(1):23–34
Zhong Y et al (2012) Induction of brain CYP2E1 by chronic ethanol treatment and related oxidative stress in hippocampus, cerebellum, and brainstem. Toxicology 302(2-3):275–284
Hansson T et al (1990) Regional distribution of ethanol-inducible cytochrome P450 IIE1 in the rat central nervous system. Neuroscience 34(2):451–463
Quertemont E et al (2005) Is ethanol a pro-drug? Acetaldehyde contribution to brain ethanol effects. Alcohol Clin Exp Res 29(8):1514–1521
Vasiliou V et al (2006) CYP2E1 and catalase influence ethanol sensitivity in the central nervous system. Pharmacogenet Genomics 16(1):51–58
Zimatkin SM et al (2006) Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res 30(9):1500–1505
Burd L, Blair J, Dropps K (2012) Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J Perinatol 32(9):652–659
Goodlett CR, Horn KH (2001) Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health 25(3):175–184
Bhatia S et al (2019) Oxidative stress and DNA damage in the mechanism of fetal alcohol spectrum disorders. Birth Defects Res 111(12):714–748
Luo J (2014) Autophagy and ethanol neurotoxicity. Autophagy 10(12):2099–2108
Kaminen-Ahola N (2020) Fetal alcohol spectrum disorders: genetic and epigenetic mechanisms. Prenat Diagn 40(9):1185–1192
Ji C (2012) Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int 2012:216450
Ji C, Kaplowitz N (2003) Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124(5):1488–1499
Lugea A et al (2011) Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 140(3):987–997
Pandol SJ et al (2010) Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig Dis 28(6):776–782
Waldron RT et al (2018) Ethanol induced disordering of pancreatic acinar cell endoplasmic reticulum: an ER stress/defective unfolded protein response model. Cell Mol Gastroenterol Hepatol 5(4):479–497
Nguyen KH, Lee JH, Nyomba BL (2012) Ethanol causes endoplasmic reticulum stress and impairment of insulin secretion in pancreatic β-cells. Alcohol 46(1):89–99
Wu H et al (2021) MANF protects pancreatic acinar cells against alcohol-induced endoplasmic reticulum stress and cellular injury. J Hepatobiliary Pancreat Sci
Srinivasan MP et al (2021) Differential cytotoxicity, ER/oxidative stress, dysregulated AMPKα signaling, and mitochondrial stress by ethanol and its metabolites in human pancreatic acinar cells. Alcohol Clin Exp Res
Li SY et al (2009) Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol 47(2):247–255
Li SY, Ren J (2008) Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol 44(6):992–1001
Wang W et al (2021) Astaxanthin attenuates alcoholic cardiomyopathy via inhibition of endoplasmic reticulum stress-mediated cardiac apoptosis. Toxicol Appl Pharmacol 412:115378
Ji C (2015) Advances and new concepts in alcohol-induced organelle stress, unfolded protein responses and organ damage. Biomolecules 5(2):1099–1121
George AK et al (2017) Hydrogen sulfide, endoplasmic reticulum stress and alcohol mediated neurotoxicity. Brain Res Bull 130:251–256
Chen G et al (2008) Ethanol promotes endoplasmic reticulum stress-induced neuronal death: involvement of oxidative stress. J Neurosci Res 86(4):937–946
Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochim Biophys Acta 1833(12):3460–3470
Ji C et al (2005) Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res 29(8):1496–1503
Lee H et al (2011) Endoplasmic reticulum stress-induced JNK activation is a critical event leading to mitochondria-mediated cell death caused by β-lapachone treatment. PLoS ONE 6(6):e21533
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Matson LM, Grahame NJ (2013) Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol 18(6):921–929
Xu H et al (2021) Chronic voluntary alcohol drinking causes anxiety-like behavior, thiamine deficiency, and brain damage of female crossed high alcohol preferring mice. Front Pharmacol 12(258)
Xu H et al (2019) Effects of chronic voluntary alcohol drinking on thiamine concentrations, endoplasmic reticulum stress, and oxidative stress in the brain of crossed high alcohol preferring mice. Neurotox Res
George AK et al (2018) Exercise mitigates alcohol induced endoplasmic reticulum stress mediated cognitive impairment through ATF6-Herp signaling. Sci Rep 8(1):5158
Cassidy LL, Dlugos FF, Dlugos CA (2013) Time course of SERCA 2b and calreticulin expression in Purkinje neurons of ethanol-fed rats with behavioral correlates. Alcohol Alcohol 48(6):667–678
Dlugos CA (2006) Smooth endoplasmic reticulum dilation and degeneration in Purkinje neuron dendrites of aging ethanol-fed female rats. Cerebellum 5(2):155–162
Dlugos CA (2014) ATF6 and caspase 12 expression in Purkinje neurons in acute slices from adult, ethanol-fed rats. Brain Res 1577:11–20
Acknowledgements
We thank Cody Dvorak for proofreading the manuscript.
Funding
This work was supported by the National Institutes of Health (NIH) grants AA017226 and AA015407. It was also supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development [Biomedical Laboratory Research and Development: Merit Review (BX001721)].
Author information
Authors and Affiliations
Contributions
W. W. reviewed literatures, wrote the manuscript, and made the tables and figures; H. L. revised the manuscript; and J. L. revised the manuscript and provided financial support. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wen, W., Li, H. & Luo, J. Potential Role of MANF, an ER Stress Responsive Neurotrophic Factor, in Protecting Against Alcohol Neurotoxicity. Mol Neurobiol 59, 2992–3015 (2022). https://doi.org/10.1007/s12035-022-02786-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02786-7