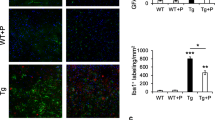
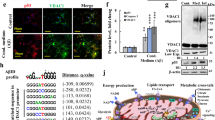
Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by cognitive dysfunction. The glutamate (Glu) metabolic pathway may be a major contributor to the memory dysfunction associated with AD. The TWIK-related potassium channel-1 (TREK-1) protects against brain ischemia, but any specific role for the channel in AD remains unknown. In this study, we used SAMP8 mice as an AD model and age-matched SAMR1 mice as controls. We explored the trends of changes in TREK-1 channel activity and the levels of AD-related molecules in the brains of SAMP8 mice. We found that the expression level of TREK-1 increased before 3 months of age and then began to decline. The levels of Tau and Glu increased with age whereas the acetylcholine level decreased with age. α-Linolenic acid (ALA), an activator of the TREK-1 channel, significantly increased the TREK-1 level, and improved the learning and memory deficits of SAMP8 mice aged 6 months. The mechanism in play may involve the Glu metabolic pathway. After activation of the TREK-1 channel, damaged neurons and astrocytes were rescued, the levels of Glu and the N-methyl-D-aspartate receptor were downregulated, and the level of glutamate transporter-1 was upregulated. These findings suggest that TREK-1 plays a crucial role in the pathological progression of AD; activation of the TREK-1 channel improved cognitive deficits in SAMP8 mice via a mechanism that involved Glu metabolism. The TREK-1 potassium channel may thus be a valuable therapeutic target in AD patients.







Similar content being viewed by others
Data Availability
Data supporting the results of this study are available upon reasonable request of the corresponding author.
Code Availability
Not applicable.
References
Goedert MS, Pillantini MG (2006) A century of Alzheimer's disease. Science 314(5800):777–781. https://doi.org/10.1126/science.1132814
Jeong S (2017) Molecular and cellular basis of neurodegeneration in Alzheimer's disease. Mol Cells 40(9):613–620. https://doi.org/10.14348/molcells.2017.0096
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer's disease: Targeting the cholinergic system. Curr Neuropharmacol 14(1):101–115. https://doi.org/10.2174/1570159x13666150716165726
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35. https://doi.org/10.1007/s00401-009-0619-8
Carozzi V, Marmiroli P, Cavaletti G (2008) Focus on the role of Glutamate in the pathology of the peripheral nervous system. CNS Neurol Disord Drug Targets 7(4):348–360. https://doi.org/10.2174/187152708786441876
Ferraguti F, Crepaldi L, Nicoletti F (2008) Metabotropic glutamate 1 receptor: current concepts and perspectives. Pharmacol Rev 60(4):536–581. https://doi.org/10.1124/pr.108.000166
Nakanishi S, Masu M (1994) Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct 23:319–348. https://doi.org/10.1146/annurev.bb.23.060194.001535
Riederer P, Hoyer S (2006) From benefit to damage. Glutamate and advanced glycation end products in Alzheimer brain. J Neural Transm (Vienna) 113(11):1671–1677. https://doi.org/10.1007/s00702-006-0591-6
Danysz W, Wroblewski JT, Costa E (1988) Learning impairment in rats by N-methyl-D-aspartate receptor antagonists. Neuropharmacology 27(6):653–656. https://doi.org/10.1016/0028-3908(88)90189-x
Morris RG (1989) Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci 9(9):3040–3057. https://doi.org/10.1523/jneurosci.09-09-03040.1989
Revett TJ, Baker GB, Jhamandas J, Kar S (2013) Glutamate system, amyloid ß peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci 38(1):6–23. https://doi.org/10.1503/jpn.110190
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27(11):2866–2875. https://doi.org/10.1523/jneurosci.4970-06.2007
Yi JH, Pow DV, Hazell AS (2005) Early loss of the glutamate transporter splice-variant GLT-1v in rat cerebral cortex following lateral fluid-percussion injury. Glia 49(1):121–133. https://doi.org/10.1002/glia.20099
Meeker KD, Meabon JS, Cook DG (2015) Partial loss of the glutamate transporter GLT-1 alters brain Akt and insulin signaling in a mouse model of Alzheimer's disease. J Alzheimers Dis 45(2):509–520. https://doi.org/10.3233/jad-142304
Chapman CG, Meadows HJ, Godden RJ, Campbell DA, Duckworth M, Kelsell RE et al (2000) Cloning, localisation and functional expression of a novel human, cerebellum specific, two pore domain potassium channel. Brain Res Mol Brain Res 82(1-2):74–83. https://doi.org/10.1016/s0169-328x(00)00183-2
Hervieu GJ, Cluderay JE, Gray CW, Green PJ, Ranson JL, Randall AD et al (2001) Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience 103(4):899–919. https://doi.org/10.1016/s0306-4522(01)00030-6
Vivier D, Bennis K, Lesage F, Ducki S (2016) Perspectives on the two-pore domain potassium channel TREK-1 (TWIK-Related K(+) Channel 1). A novel therapeutic target? J Med Chem 59(11):5149–5157. https://doi.org/10.1021/acs.jmedchem.5b00671
Yarishkin O, Phuong TTT, Bretz CA, Olsen KW, Baumann JM, Lakk M et al (2018) TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. J Gen Physiol 150(12):1660–1675. https://doi.org/10.1085/jgp.201812179
Goonetilleke L, Quayle J (2012) TREK-1 K(+) channels in the cardiovascular system: their significance and potential as a therapeutic target. Cardiovasc Ther 30(1):e23–e29. https://doi.org/10.1111/j.1755-5922.2010.00227.x
Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M et al (2004) TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J 23(13):2684–2695. https://doi.org/10.1038/sj.emboj.7600234
Liu Y, Sun Q, Chen X, Jing L, Wang W, Yu Z et al (2014) Linolenic acid provides multi-cellular protective effects after photothrombotic cerebral ischemia in rats. Neurochem Res 39(9):1797–1808. https://doi.org/10.1007/s11064-014-1390-3
Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y (2014) Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol 12(1):e1001747. https://doi.org/10.1371/journal.pbio.1001747
Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY et al (2012) TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151(1):25–40. https://doi.org/10.1016/j.cell.2012.09.005
Lu L, Zhang G, Song C, Wang X, Qian W, Wang Z et al (2017) Arachidonic acid has protective effects on oxygen-glucose deprived astrocytes mediated through enhancement of potassium channel TREK-1 activity. Neurosci Lett 636:241–247. https://doi.org/10.1016/j.neulet.2016.11.034
Bittner S, Ruck T, Schuhmann MK, Herrmann AM, Moha ou Maati H, Bobak N et al (2013) Endothelial TWIK-related potassium channel-1 (TREK1) regulates immune-cell trafficking into the CNS. Nat Med 19(9):1161–1165. https://doi.org/10.1038/nm.3303
Akiguchi I, Pallàs M, Budka H, Akiyama H, Ueno M, Han J et al (2017) SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda's legacy and future directions. Neuropathology 37(4):293–305. https://doi.org/10.1111/neup.12373
Orejana L, Barros-Miñones L, Jordán J, Puerta E, Aguirre N (2012) Sildenafil ameliorates cognitive deficits and tau pathology in a senescence-accelerated mouse model. Neurobiol Aging 33(3):625.e611–625.e620. https://doi.org/10.1016/j.neurobiolaging.2011.03.018
Diaz-Perdigon T, Belloch FB, Ricobaraza A, Elboray EE, Suzuki T, Tordera RM et al (2020) Early sirtuin 2 inhibition prevents age-related cognitive decline in a senescence-accelerated mouse model. Neuropsychopharmacology 45(2):347–357. https://doi.org/10.1038/s41386-019-0503-8
Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M (2000) Polyunsaturated fatty acids are potent neuroprotectors. EMBO J 19(8):1784–1793. https://doi.org/10.1093/emboj/19.8.1784
Djillani A, Mazella J, Heurteaux C, Borsotto M (2019) Role of TREK-1 in health and disease, focus on the central nervous system. Front Pharmacol 10:379. https://doi.org/10.3389/fphar.2019.00379
Lin R, Li L, Zhang Y, Huang S, Chen S, Shi J et al (2018) Electroacupuncture ameliorate learning and memory by improving N-acetylaspartate and glutamate metabolism in APP/PS1 mice. Biol Res 51(1):21. https://doi.org/10.1186/s40659-018-0166-7
Gao Y, Tan L, Yu JT, Tan L (2018) Tau in Alzheimer's disease: mechanisms and therapeutic strategies. Curr Alzheimer Res 15(3):283–300. https://doi.org/10.2174/1567205014666170417111859
Miyasaka T, Shinzaki Y, Yoshimura S, Yoshina S, Kage-Nakadai E, Mitani S et al (2018) Imbalanced expression of tau and tubulin induces neuronal dysfunction in C. elegans Models of Tauopathy. Front Neurosci 12:415. https://doi.org/10.3389/fnins.2018.00415
Tsolaki M, Sakka V, Gerasimou G, Dimacopoulos N, Chatzizisi O, Fountoulakis KN et al (2001) Correlation of rCBF (SPECT), CSF tau, and cognitive function in patients with dementia of the Alzheimer's type, other types of dementia, and control subjects. Am J Alzheimers Dis Other Demen 16(1):21–31. https://doi.org/10.1177/153331750101600107
Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW et al (2010) Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67(6):953–966. https://doi.org/10.1016/j.neuron.2010.08.044
Smith JW, Evans AT, Costall B, Smythe JW (2002) Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev 26(1):45–60. https://doi.org/10.1016/s0149-7634(01)00037-9
Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G et al (2010) Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci 13(4):411–413. https://doi.org/10.1038/nn.2511
Hascup KN, Hascup ER, Pomerleau F, Huettl P, Gerhardt GA (2008) Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J Pharmacol Exp Ther 324(2):725–731. https://doi.org/10.1124/jpet.107.131698
Sun Y, Yang J, Hu X, Gao X, Li Y, Yu M et al (2018) Conditioned medium from overly excitatory primary astrocytes induced by La(3+) increases apoptosis in primary neurons via upregulating the expression of NMDA receptors. Metallomics 10(7):1016–1028. https://doi.org/10.1039/c8mt00056e
Mahmoud S, Gharagozloo M, Simard C, Gris D (2019) Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 8(2). https://doi.org/10.3390/cells8020184
Gu B, Nakamichi N, Zhang WS, Nakamura Y, Kambe Y, Fukumori R et al (2009) Possible protection by notoginsenoside R1 against glutamate neurotoxicity mediated by N-methyl-D-aspartate receptors composed of an NR1/NR2B subunit assembly. J Neurosci Res 87(9):2145–2156. https://doi.org/10.1002/jnr.22021
Mookherjee P, Green PS, Watson GS, Marques MA, Tanaka K, Meeker KD et al (2011) GLT-1 loss accelerates cognitive deficit onset in an Alzheimer's disease animal model. J Alzheimers Dis 26(3):447–455. https://doi.org/10.3233/jad-2011-110503
Acknowledgements
We thank the members of the Lu Li Laboratory, Institute of Pharmacology, Lanzhou University School of Basic Medicine, for their constructive feedback on the production of this manuscript.
Funding
This study was supported by the Key Laboratory of Traditional Chinese Medicine Innovation and Transformation of Gansu Province/Engineering Laboratory of Traditional Chinese Medicine Products of Gansu Province (ZYFYZH-KJ-2016–004) and the Talent Innovation and Entrepreneurial Science and Technology Project of Lanzhou City (2015-RC-20).
Author information
Authors and Affiliations
Contributions
F Li has full access to all data in the study and is responsible for the integrity of the data and the accuracy of the data analysis. SN Zhou and F Li contributed equally to this work and are co-first authors. F Li collected the data and drafted and revised the manuscript. SN Zhou analyzed the data. X Zeng did animal experiments, Z Li finished ELISA detection, and R Yang and XX Wang contributed to section staining. B Meng and WL Pei collected the data. L Li designed the study and F Li interpreted data and reviewed the manuscript. L Li and XX Wang did critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
All the animal experiments were conducted according to the guidelines for animal care and use in China. The authors also confirm that the study was performed in accordance with the approval of the ethical committee for animal experimentation at Lanzhou University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fang Li and Shu-ning Zhou are co-first authors.
Li Lu is the first corresponding author and Rui Yang is the second corresponding author.
Rights and permissions
About this article
Cite this article
Li, F., Zhou, Sn., Zeng, X. et al. Activation of the TREK-1 Potassium Channel Improved Cognitive Deficits in a Mouse Model of Alzheimer’s Disease by Modulating Glutamate Metabolism. Mol Neurobiol 59, 5193–5206 (2022). https://doi.org/10.1007/s12035-022-02776-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02776-9