Abstract
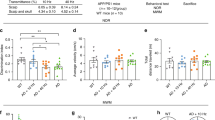
It has been described that using noninvasive exposure to 40-Hz white light LED reduces amyloid-beta, a peptide thought to initiate neurotoxic events in Alzheimer’s disease (AD). However, the mechanisms remain to be identified. Since AD impairs mitochondrial potassium channels and respiratory chain activity, the objectives of the current study were to determine the effect of 40-Hz white light LED on structure–function of mitoKATP channel and brain mitochondrial respiratory chain activity, production of reactive oxygen species (ROS), and ΔΨm in AD. Single mitoKATP channel was considered using a channel incorporated into the bilayer lipid membrane and expression of mitoKATP-Kir6.1 subunit as a pore-forming subunit of the channel was determined using a western blot analysis in Aβ1-42 toxicity and light-treated rats. Our results indicated a severe decrease in mito-KATP channel permeation and Kir6.1 subunit expression coming from the Aβ1-42-induced neurotoxicity. Furthermore, we found that Aβ1-42-induced neurotoxicity decreased activities of complexes I and IV and increased ROS production and ΔΨm. Surprisingly, light therapy increased channel permeation and mitoKATP-Kir6.1 subunit expression. Noninvasive 40-Hz white light LED treatment also increased activities of complexes I and IV and decreased ROS production and ΔΨm up to ~ 70%. Here, we report that brain mito-KATP channel and respiratory chain are, at least in part, novel targets of 40-Hz white light LED therapy in AD.








Similar content being viewed by others
Data Availability
Data and material are available upon request to the corresponding author.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Beta amyloid
- PBMT:
-
Photobiomodulation therapy
- LED:
-
Light-emitting diode
- MitoKATP channel:
-
Mitochondrial ATP-sensitive potassium channel
- ROS:
-
Reactive oxygen species
- ΔΨm :
-
Mitochondrial membrane potential
- ICV:
-
Intracerebroventricular
- STL:
-
Step-through latency
- TDC:
-
Time spent in the dark compartment
- TLC:
-
Thin-layer chromatography
- BLM:
-
Bilayer lipid membrane
- COX:
-
Cytochrome oxidase
- ETC:
-
Electron transport chain
- Complex I:
-
NADH-CoQ oxidoreductase
- Complex IV:
-
Cytochrome c oxidase
- CoQ:
-
Coenzyme Q
- DCFH-DA:
-
2′,7′-Dichlorofluorescein diacetate
- Rh 123:
-
Rhodamine 123
- Po:
-
Open probability
- V 1/2 :
-
Voltage for half-maximal activation
- z d :
-
Equivalent gating charge
References
Cornejo VH, Hetz C, editors (2013) The unfolded protein response in Alzheimer’s disease. Seminars in immunopathology. Springer. https://doi.org/10.1007/s00281-013-0373-9
Cummings JL, Morstorf T, Zhong K (2014) Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Res Ther 6:1–7
Salloway S, Sperling R, Fox NC et al (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370:322–333. https://doi.org/10.1056/NEJMoa1304839
Lane N (2006) Power games. Nature Publishing Group, Berlin
Hamblin MR (2016) Shining light on the head: photobiomodulation for brain disorders. BBA Clin 6:113–124. https://doi.org/10.1016/j.bbacli.2016.09.002
Dougal G, Lee S (2013) Evaluation of the efficacy of low-level light therapy using 1072 nm infrared light for the treatment of herpes simplex labialis. Clin Exp Dermatol 38:713–718. https://doi.org/10.1111/ced.12069
Vatansever F, Hamblin MR (2012) Far infrared radiation (FIR): its biological effects and medical applications: Ferne Infrarotstrahlung: Biologische Effekte und medizinische Anwendungen. Photonics lasers Med 1:255–266. https://doi.org/10.1515/plm-2012-0034
Hashmi JT, Huang YY, Sharma SK et al (2010) Effect of pulsing in low-level light therapy. Lasers Surg Med 42:450–466. https://doi.org/10.1002/lsm.20950
Hamblin MR, editor (2019) Photobiomodulation for Alzheimer’s disease: has the light dawned? Photonics. Multidisciplinary Digital Publishing Institute, Basel. https://doi.org/10.3390/photonics6030077
Rojas JC, Gonzalez-Lima F (2013) Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol 86:447–457. https://doi.org/10.1016/j.bcp.2013.06.012
Iaccarino HF, Singer AC, Martorell AJ et al (2016) Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540:230–235. https://doi.org/10.1038/nature20587
Singer AC, Martorell AJ, Douglas JM et al (2018) Noninvasive 40-Hz light flicker to recruit microglia and reduce amyloid beta load. Nat Protoc 13:1850–1868. https://doi.org/10.1038/s41596-018-0021-x
Lu Y, Wang R, Dong Y et al (2017) Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging 49:165–182. https://doi.org/10.1016/j.neurobiolaging.2016.10.003
Wataha J, Lewis J, Lockwood P et al (2004) Blue light differentially modulates cell survival and growth. J Dental Res 83:104–108. https://doi.org/10.1177/154405910408300204
Gorgidze L, Oshemkova S, Vorobjev I (1998) Blue light inhibits mitosis in tissue culture cells. Biosci Rep 18:215–224. https://doi.org/10.1023/A:1020104914726
García-Silva MT, Ribes A, Campos Y et al (1997) Syndrome of encephalopathy, petechiae, and ethylmalonic aciduria. Pediatr Neurol 17:165–170
Hockberger PE, Skimina TA, Centonze VE et al (1999) Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc Natl Acad Sci 96:6255–6260. https://doi.org/10.1073/pnas.96.11.6255
Devi L, Prabhu BM, Galati DF et al (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci 26:9057–9068. https://doi.org/10.1523/JNEUROSCI.1469-06.2006
Correia SC, Carvalho C, Cardoso S et al (2010) Mitochondrial preconditioning: a potential neuroprotective strategy. Front Aging Neurosci 2:138. https://doi.org/10.3389/fnagi.2010.00138
Valla J, Schneider L, Niedzielko T et al (2006) Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion 6:323–330. https://doi.org/10.1016/j.mito.2006.10.004
Bosetti F, Brizzi F, Barogi S et al (2002) Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol Aging 23:371–376. https://doi.org/10.1016/S0197-4580(01)00314-1
Choma K, Bednarczyk P, Koszela-Piotrowska I et al (2009) Single channel studies of the ATP-regulated potassium channel in brain mitochondria. J Bioenerg Biomembr 41:323. https://doi.org/10.1007/s10863-009-9233-7
Ma G, Chen S (2004) Diazoxide and Nω-nitro-L-arginine counteracted Aβ1-42-induced cytotoxicity. NeuroReport 15:1813–1817. https://doi.org/10.1097/01.wnr.0000135694.89237.3d
Alejandro A, S. Eliza H, Colin GN, Monica SR (2009) Molecular biology of KATP channels and implications for health and disease. IUBMB life 61:971–978. https://doi.org/10.1002/iub.246
Szabo I, Zoratti M (2014) Mitochondrial channels: ion fluxes and more. Physiol Rev 94:519–608. https://doi.org/10.1152/physrev.00021.2013
Peng K, Hu J, Xiao J et al (2018) Mitochondrial ATP-sensitive potassium channel regulates mitochondrial dynamics to participate in neurodegeneration of Parkinson’s disease. BBA-Mol Basis Dis 1864:1086–1103. https://doi.org/10.1016/j.bbadis.2018.01.013
Jafari A, Noursadeghi E, Khodagholi F et al (2015) Brain mitochondrial ATP-insensitive large conductance Ca+ 2-activated K+ channel properties are altered in a rat model of amyloid-β neurotoxicity. Exp Neurol 269:8–16. https://doi.org/10.1016/j.expneurol.2014.12.024
Boyd-Kimball D, Sultana R, Poon HF et al (2005) Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid β-peptide (1–42) into rat brain: implications for Alzheimer’s disease. Neuroscience 132:313–324. https://doi.org/10.1016/j.neuroscience.2004.12.022
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates: hard, cover. Elsevier, Amsterdam
Davoodi FG, Motamedi F, Akbari E et al (2011) Effect of reversible inactivation of reuniens nucleus on memory processing in passive avoidance task. Behavi Brain Res 221:1–6. https://doi.org/10.1016/j.bbr.2011.02.020
Rosenthal RE, Hamud F, Fiskum G et al (1987) Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab 7:752–758. https://doi.org/10.1038/jcbfm.1987.130
Da Cruz S, Xenarios I, Langridge J et al (2003) Proteomic analysis of the mouse liver mitochondrial inner membrane. J Biol Chem 278:41566–41571. https://doi.org/10.1074/jbc.M304940200
Singleton W, Gray M, Brown M, White J (1965) Chromatographically homogeneous lecithin from egg phospholipids. J Am Oil Chem Soc 42:53–56. https://doi.org/10.1007/BF02558256
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/j.neuroscience.2004.12.022
Torabi N, Noursadeghi E, Shayanfar F et al (2021) Intranasal insulin improves the structure–function of the brain mitochondrial ATP–sensitive Ca2+ activated potassium channel and respiratory chain activities under diabetic conditions. BBA-Mol Basis Dis 1867:166075. https://doi.org/10.1016/j.bbadis.2021.166075
Thapa D, Nichols CE, Lewis SE et al (2015) Transgenic overexpression of mitofilin attenuates diabetes mellitus-associated cardiac and mitochondria dysfunction. J Mol Cell Cardiol 79:212–223. https://doi.org/10.1016/j.yjmcc.2014.11.008
Baseler WA, Dabkowski ER, Jagannathan R et al (2013) Reversal of mitochondrial proteomic loss in type 1 diabetic heart with overexpression of phospholipid hydroperoxide glutathione peroxidase. Am J Physiol-Regul, Integr Comp Physiol 304:R553–R565. https://doi.org/10.1152/ajpregu.00249.2012
Fahanik-Babaei J, Eliassi A, Jafari A et al (2011) Electro-pharmacological profile of a mitochondrial inner membrane big-potassium channel from rat brain. BBA-Biomembr 1808:454–460. https://doi.org/10.1016/j.bbamem.2010.10.005
Fahanik-Babaei J, Rezaee B, Nazari M et al (2020) A new brain mitochondrial sodium-sensitive potassium channel: effect of sodium ions on respiratory chain activity. J Cell Sci 133:jcs242446. https://doi.org/10.1242/jcs.242446
Navarro A, Gómez C, Sánchez-Pino M-J et al (2005) Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol-Regul, Integr Comp Physiol 289:R1392–R1399. https://doi.org/10.1152/ajpregu.00834.2004
Spinazzi M, Casarin A, Pertegato V et al (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat protoc 7:1235. https://doi.org/10.1038/nprot.2012.058
Luo J, Shi R (2005) Acrolein induces oxidative stress in brain mitochondria. Neurochem Int 46:243–252. https://doi.org/10.1016/j.neuint.2004.09.001
Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 153:329–338. https://doi.org/10.1210/en.2011-1502
Lacza Z, Snipes JA, Kis B et al (2003) Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Res 994:27–36. https://doi.org/10.1016/j.brainres.2003.09.046
Brustovetsky T, Shalbuyeva N, Brustovetsky N (2005) Lack of manifestations of diazoxide/5-hydroxydecanoate-sensitive KATP channel in rat brain nonsynaptosomal mitochondria. J Physiol 568:47–59. https://doi.org/10.1113/jphysiol.2005.091199
Moreira PI, Zhu X, Wang X et al (2010) Mitochondria: a therapeutic target in neurodegeneration. BBA-Mol Basis Dis 1802:212–220. https://doi.org/10.1016/j.bbadis.2009.10.007
Paggio A, Checchetto V, Campo A et al (2019) Identification of an ATP-sensitive potassium channel in mitochondria. Nature 572:609–613. https://doi.org/10.1038/s41586-019-1498-3
Inoue I, Nagase H, Kishi K, Higuti T (1991) ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 352:244–247
Liu D, Pitta M, Lee J-H et al (2010) The K ATP channel activator diazoxide ameliorates amyloid-β and Tau pathologies and improves memory in the 3xTgAD mouse model of Alzheimer’s disease. J Alzheimers Dis 22:443–457. https://doi.org/10.3233/JAD-2010-101017
Wu L, Shen F, Lin L et al (2006) The neuroprotection conferred by activating the mitochondrial ATP-sensitive K+ channel is mediated by inhibiting the mitochondrial permeability transition pore. Neurosci Lett 402:184–189. https://doi.org/10.1016/j.neulet.2006.04.001
Liu D, Lu C, Wan R et al (2002) Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab 22:431–443. https://doi.org/10.1097/00004647-200204000-00007
Macauley SL, Stanley M, Caesar EE et al (2015) Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J Clin Investig 125:2463–2467
Xie J, Duan L, Qian X et al (2010) KATP channel openers protect mesencephalic neurons against MPP+-induced cytotoxicity via inhibition of ROS production. J Neurosci Res 88:428–437. https://doi.org/10.1002/jnr.22213
Virgili N, Mancera P, Wappenhans B et al (2013) KATP channel opener diazoxide prevents neurodegeneration: a new mechanism of action via antioxidative pathway activation. PLoS ONE 8:e75189. https://doi.org/10.1371/annotation/0e045706-ea24-41db-be90-27d1cbcd35b1
Gozen A, Demiryurek S, Taskin A et al (2013) Protective activity of ischemic preconditioning on rat testicular ischemia: effects of Y-27632 and 5-hydroxydecanoic acid. J Pediatr Surg 48:1565–1572. https://doi.org/10.1016/j.jpedsurg.2012.10.074
Tosun C, Koltz MT, Kurland DB et al (2013) The protective effect of glibenclamide in a model of hemorrhagic encephalopathy of prematurity. Brain Sci 3:215–238. https://doi.org/10.3390/brainsci3010215
Erejuwa OO, Sulaiman SA, Wahab MSA et al (2010) Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int J Mol Sci 11:2056–2066. https://doi.org/10.3390/ijms11052056
Atlasz T, Babai N, Reglodi D et al (2007) Diazoxide is protective in the rat retina against ischemic injury induced by bilateral carotid occlusion and glutamate-induced degeneration. Neurotox Res 12:105–111. https://doi.org/10.1007/BF03033919
Hu P, Zheng M, Jiang J et al (2010) Effects of diazoxide on the mitochondrial ultrastructure and permeability in donor rat myocardium. Zhongguo ying yong sheng li xue za zhi= Zhongguo yingyong shenglixue zazhi. Chin J Appl Physiol 26:19–22
Nesi G, Sestito S, Digiacomo M, Rapposelli S (2017) Oxidative stress, mitochondrial abnormalities and proteins deposition: multitarget approaches in Alzheimer’s disease. Curr Top Med Chem 17:3062–3079. https://doi.org/10.2174/1568026617666170607114232
Tiiman A, Palumaa P, Tougu V (2013) The missing link in the amyloid cascade of Alzheimer’s disease—metal ions. Neurochem Int 62:367–378. https://doi.org/10.1016/j.neuint.2013.01.023
Flannery PJ, Trushina E (2019) Mitochondrial dysfunction in Alzheimer’s disease and progress in mitochondria-targeted therapeutics. Curr Behav Neurosci Rep 6:88–102. https://doi.org/10.1007/s40473-019-00179-0
Trushina E, McMurray C (2007) Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience 145:1233–1248. https://doi.org/10.1016/j.neuroscience.2006.10.056
Kilbride SM, Gluchowska SA, Telford JE et al (2011) High-level inhibition of mitochondrial complexes III and IV is required to increase glutamate release from the nerve terminal. Mol Neurodegener 6:1–9. https://doi.org/10.1186/1750-1326-6-53
Golpich M, Amini E, Mohamed Z et al (2017) Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Therap 23:5–22. https://doi.org/10.1111/cns.12655
Murphy PM (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13. https://doi.org/10.1042/BJ20081386
Scialò F, Fernández-Ayala DJ, Sanz A (2017) Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front Physiol 8:428. https://doi.org/10.3389/fphys.2017.00428
Dong Y, Digman MA, Brewer GJ (2019) Age-and AD-related redox state of NADH in subcellular compartments by fluorescence lifetime imaging microscopy. Geroscience 41:51–67. https://doi.org/10.1007/s11357-019-00052-8
Hajnóczky G, Csordás G, Madesh M, Pacher P (2000) The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J Physiol 529:69–81. https://doi.org/10.1111/j.1469-7793.2000.00069.x
Mellerio J (1994) Light effects on the retina. Principles and practice of ophthalmology 1:1326–1345
García J, Silva E (1997) Flavin-sensitized photooxidation of amino acids present in a parenteral nutrition infusate: protection by ascorbic acid. J Nutr Biochem 8:341–345. https://doi.org/10.1016/S0955-2863(97)00024-7
Verret L, Mann EO, Hang GB et al (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149:708–721. https://doi.org/10.1016/j.cell.2012.02.046
Gillespie AK, Jones EA, Lin Y-H et al (2016) Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron 90:740–751. https://doi.org/10.1016/j.neuron.2016.04.009
Lu CB, Vreugdenhil M, Toescu EC (2012) The effect of aging-associated impaired mitochondrial status on kainate-evoked hippocampal gamma oscillations. Neurobiol Aging 33:2692–2703. https://doi.org/10.1016/j.neurobiolaging.2012.01.001
Whittaker RG, Turnbull DM, Whittington MA, Cunningham MO (2011) Impaired mitochondrial function abolishes gamma oscillations in the hippocampus through an effect on fast-spiking interneurons. Brain 134:e180. https://doi.org/10.1093/brain/awr018
Kann O, Huchzermeyer C, Kovacs R et al (2011) Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain 134:345–358. https://doi.org/10.1093/brain/awq333
Malinska D, Kulawiak B, Kudin AP et al (2010) Complex III-dependent superoxide production of brain mitochondria contributes to seizure-related ROS formation. Biochim Biophys Acta. https://doi.org/10.1016/j.bbabio.2010.03.001
Malinska D, Mirandola SR, Kunz WS (2010) Mitochondrial potassium channels and reactive oxygen species. FEBS Lett. https://doi.org/10.1016/j.febslet.2010.01.013
Szewczyk A, Kajma A, Malinska D et al (2010) Pharmacology of mitochondrial potassium channels: dark side of the field. FEBS Lett. https://doi.org/10.1016/j.febslet.2010.02.048
Busija DW, Katakam P, Rajapakse NC et al (2005) Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Res Bull 66:85–90. https://doi.org/10.1016/j.brainresbull.2005.03.022
Ardehali H, Chen Z, Ko Y et al (2004) Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci 101:11880–11885. https://doi.org/10.1073/pnas.0401703101
Wojtovich AP, Brookes PS (2008) The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic. BBA-Bioenerg 1777:882–889. https://doi.org/10.1016/j.bbabio.2008.03.025
Wojtovich AP, Nehrke KW, Brookes PS (2010) The mitochondrial complex II and ATP-sensitive potassium channel interaction: quantitation of the channel in heart mitochondria. Acta Biochim Pol 57:431 (PMID: 21103454)
Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD (2001) Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol Heart Circ Physiol 280:H649–H657. https://doi.org/10.1152/ajpheart.2001.280.2.H649
Funding
This work was supported by a grant from the Neurophysiology Research Center of Shahid Beheshti University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
M.N.: investigation, formal analysis, resources, writing original draft; T.V-S.: software, resources; NT: formal analysis, resources; J.F-B: formal analysis; R.S.: resources; F.K.: resources, project administration; A.E.: conceptualization, supervision, writing—review and editing, project administration.
Corresponding author
Ethics declarations
Ethics Approval
All experiments were conducted according to the Guide for Care and Use of Laboratory Animals (National Institutes of Health Publication No. 80–23, revised 1996). Additionally, all procedures were reviewed and confirmed by the Research and Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1398.109).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Structure–function of brain mitoKATP channel is severely damaged in an Aβ toxicity model.
• Our study suggests certain mechanisms account for the effects of Aβ toxicity on mitochondria.
• Forty-Hertz white light therapy modifies mitoKATP structure–function in Aβ toxicity rats.
• Forty-Hertz white light improves respiratory chain activity, ROS, and ΔΨm in Aβ toxicity rats.
• Our study suggests a mechanism for the efficacy of 40-Hz light therapy in Aβ toxicity rats.
Rights and permissions
About this article
Cite this article
Nazari, M., Vajed-Samiei, T., Torabi, N. et al. The 40-Hz White Light-Emitting Diode (LED) Improves the Structure–Function of the Brain Mitochondrial KATP Channel and Respiratory Chain Activities in Amyloid Beta Toxicity. Mol Neurobiol 59, 2424–2440 (2022). https://doi.org/10.1007/s12035-021-02681-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02681-7