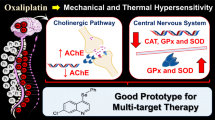
Abstract
Almost 90% of patients develop pain immediately after oxaliplatin (OXA) treatment. Here, the impact of aging on OXA-induced acute peripheral neuropathy and the potential of 7-chloro-4-(phenylselanyl) quinoline (4-PSQ) as a new therapeutic strategy were evaluated. In Swiss mice, the oxidative damage and its influence on Mg2+—ATPase and Na+, K+—ATPase activities were investigated. The relationship between the reactive oxygen species (ROS) and nitrate and nitrite (NOx) levels, the activity of glutathione peroxidase (GPx), and superoxide dismutase (SOD) with the development of OXA-induced acute peripheral neuropathy was also studied. In this study, it was evidenced that OXA-induced acute peripheral neuropathy was exacerbated by aging through increased oxidative damage as well as Na+, K+—ATPase, and Mg+2—ATPase inhibition. 4-PSQ reversed hypersensitivity induced by OXA and aging-aggravated by reducing ROS and NOx levels, through modulation of GPx and SOD activities. 4-PSQ partially reestablish Na+, K+—ATPase activity, but not Mg 2+—ATPase activity. Locomotor and exploratory activities were not affected. This study is the first of its kind, providing new insight into the aging impact on mechanisms involved in OXA-induced acute peripheral neuropathy. Also, it provides evidence on promising 4-PSQ effects on this condition, mainly on aging.
Graphical abstract









Similar content being viewed by others
Availability of Data and Material
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
Abbreviations
- 4-PSQ:
-
7-Chloro-4-(phenylselanyl) quinoline
- OXA:
-
Oxaliplatin
- NMDA:
-
N-Methyl-D-aspartate
- ROS:
-
Reactive oxygen species
- GPx:
-
Glutathione peroxidase
- SOD:
-
Superoxide dismutase
- NOx:
-
Nitrate and nitrite
- CNS:
-
Central nervous system
- NADPH:
-
β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
References
Nichetti F, Falvella FS, Miceli R et al (2019) Is a pharmacogenomic panel useful to estimate the risk of oxaliplatin-related neurotoxicity in colorectal cancer patients? Pharmacogenomics J. https://doi.org/10.1038/s41397-019-0078-0
Rimola V, Hahnefeld L, Zhao J et al (2020) Lysophospholipids contribute to oxaliplatin-induced acute peripheral pain. J Neurosci 40:9519–9532. https://doi.org/10.1523/JNEUROSCI.1223-20.2020
Cavaletti G, Marmiroli P (2020) Management of oxaliplatin-induced peripheral sensory neuropathy. Cancers (Basel) 12(6):1370. https://doi.org/10.3390/cancers12061370
Kawashiri T, Mine K, Kobayashi D et al (2021) Therapeutic agents for oxaliplatin-induced peripheral neuropathy; experimental and clinical evidence. Int J Mol Sci 22:1–25
Yildirim N, Cengiz M (2020) Predictive clinical factors of chronic peripheral neuropathy induced by oxaliplatin. Support Care Cancer 28:4781–4788
Kang L, Tian Y, Xu S, Chen H (2020) Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J Neurol. https://doi.org/10.1007/s00415-020-09942-w
Bennett GJ, Doyle T, Salvemini D (2014) Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol 10:326–336. https://doi.org/10.1038/NRNEUROL.2014.77
Reis AS, Paltian JJ, Domingues WB et al (2020) Advances in the understanding of oxaliplatin-induced peripheral neuropathy in mice: 7-chloro-4-(phenylselanyl) quinoline as a promising therapeutic agent. Mol Neurobiol 57:5219–5234. https://doi.org/10.1007/s12035-020-02048-4
Cavaletti G, Marmiroli P (2020) Management of oxaliplatin-induced peripheral sensory neuropathy. Cancers (Basel) 12(6):1370. https://doi.org/10.3390/cancers12061370
Fane M, Weeraratna AT (2020) How the ageing microenvironment influences tumour progression. Nat Rev Cancer 20:89–106
Ferlay J, Colombet M, Soerjomataram I et al (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144:1941–1953
Bhadra M, Howell P, Dutta S et al (2020) Alternative splicing in aging and longevity. Hum Genet 139:357–369. https://doi.org/10.1007/s00439-019-02094-6
Nguyen LD, Ehrlich BE Cellular mechanisms and treatments for chemobrain: insight from aging and neurodegenerative diseases. https://doi.org/10.15252/emmm.202012075
Liguori I, Russo G, Curcio F et al (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772. https://doi.org/10.2147/CIA.S158513
Kinoshita PF, Leite JA, Orellana AMM et al (2016) The influence of Na+, K+-ATPase on glutamate signaling in neurodegenerative diseases and senescence. Front Physiol 7:1–19. https://doi.org/10.3389/fphys.2016.00195
Shrivastava AN, Triller A, Melki R (2020) Cell biology and dynamics of Neuronal Na+/K+-ATPase in health and diseases. Neuropharmacology 169. https://doi.org/10.1016/j.neuropharm.2018.12.008
Reis AS, Paltian JJ, Domingues WB et al (2020) Pharmacological modulation of Na+, K+-ATPase as a potential target for OXA-induced neurotoxicity: Correlation between anxiety and cognitive decline and beneficial effects of 7-chloro-4-(phenylselanyl) quinoline. Brain Res Bull 162:282–290. https://doi.org/10.1016/j.brainresbull.2020.06.021
Rodrigues KC, Bortolatto CF, da Motta KP et al (2021) The neurotherapeutic role of a selenium-functionalized quinoline in hypothalamic obese rats. Psychopharmacology. https://doi.org/10.1007/s00213-021-05821-y
Vogt AG, Voss GT, de Oliveira RL et al (2018) Organoselenium group is critical for antioxidant activity of 7-chloro-4-phenylselenyl-quinoline. Chem Biol Interact 282:7–12. https://doi.org/10.1016/j.cbi.2018.01.003
Voss GT, Oliveira RL, de Souza JF et al (2018) Therapeutic and technological potential of 7-chloro-4-phenylselanyl quinoline for the treatment of atopic dermatitis-like skin lesions in mice. Mater Sci Eng C 84:90–98. https://doi.org/10.1016/j.msec.2017.11.026
Barth A, Vogt AG, dos Reis AS et al (2019) 7-Chloro-4-(phenylselanyl) quinoline with memory enhancer action in aging rats: modulation of neuroplasticity, acetylcholinesterase activity, and cholesterol levels. Mol Neurobiol. https://doi.org/10.1007/s12035-019-1530-5
da Motta KP, Lemos BB, Paltian JJ et al (2021) 7-Chloro-4-(phenylselanyl) quinoline reduces renal oxidative stress induced by oxaliplatin in mice. Can J Physiol Pharmacol. https://doi.org/10.1139/CJPP-2021-0090
Lemos BB, Motta KP da, Paltian JJ, et al (2020) Role of 7-chloro-4-(phenylselanyl) quinoline in the treatment of oxaliplatin-induced hepatic toxicity in mice. Can J Physiol Pharmacol 1–11. https://doi.org/10.1139/cjpp-2020-0134
Luchese C, Vogt AG, Pinz MP et al (2020) Amnesia-ameliorative effect of a quinoline derivative through regulation of oxidative/cholinergic systems and Na+/K+-ATPase activity in mice. Metab Brain Dis 35:589–600. https://doi.org/10.1007/s11011-020-00535-0
Paltian JJ, dos Reis AS, de Oliveira RL, et al (2020) The anxiolytic effect of a promising quinoline containing selenium with the contribution of the serotonergic and GABAergic pathways: modulation of parameters associated with anxiety in mice. Behav Brain Res 393. https://doi.org/10.1016/j.bbr.2020.112797
Pinz M, Reis AS, Duarte V et al (2016) 4-Phenylselenyl-7-chloroquinoline, a new quinoline derivative containing selenium, has potential antinociceptive and anti-inflammatory actions. Eur J Pharmacol 780:122–128. https://doi.org/10.1016/j.ejphar.2016.03.039
Savegnago L, Vieira AI, Seus N et al (2013) Synthesis and antioxidant properties of novel quinoline-chalcogenium compounds. Tetrahedron Lett 54:40–44. https://doi.org/10.1016/j.tetlet.2012.10.067
Duarte LFB, Barbosa ES, Oliveira RL et al (2017) A simple method for the synthesis of 4-arylselanyl-7-chloroquinolines used as in vitro acetylcholinesterase inhibitors and in vivo memory improvement. Tetrahedron Lett 58:3319–3322. https://doi.org/10.1016/j.tetlet.2017.07.039
Salgueiro WG, Goldani BS, Peres TV et al (2017) Insights into the differential toxicological and antioxidant effects of 4-phenylchalcogenil-7-chloroquinolines in Caenorhabditis elegans. Free Radic Biol Med 110:133–141. https://doi.org/10.1016/j.freeradbiomed.2017.05.020
de Freitas CS, Araujo SM, Bortolotto VC et al (2019) 7-chloro-4-(phenylselanyl) quinoline prevents dopamine depletion in a Drosophila melanogaster model of Parkinson’s-like disease. J Trace Elem Med Biol 54:232–243. https://doi.org/10.1016/j.jtemb.2018.10.015
de Aquino Silva D, Silva MRP, Guerra GP, et al (2021) 7-chloro-4-(phenylselanyl) quinoline co-treatment prevent oxidative stress in diabetic-like phenotype induced by hyperglycidic diet in Drosophila melanogaster. Comp Biochem Physiol Part - C Toxicol Pharmacol 239. https://doi.org/10.1016/j.cbpc.2020.108892
Guide for the Care and Use of Laboratory Animals (2011) National research council (US) committee for the update of the guide for the care and use of laboratory animals. 8th edition. National Academies Press (US), Washington (DC)
Brusco I, Justino AB, Silva CR et al (2019) Kinins and their B1 and B2 receptors are involved in fibromyalgia-like pain symptoms in mice. Biochem Pharmacol 168:119–132. https://doi.org/10.1016/j.bcp.2019.06.023
Bobinski F, Teixeira JM, Sluka KA et al (2019) HHS public access. 159:437–450. https://doi.org/10.1097/j.pain.0000000000001109.IL-4
Alamri FF, Al SA, Biggers A et al (2018) Applicability of the grip strength and automated von Frey tactile sensitivity tests in the mouse photothrombotic model of stroke. Behav Brain Res 336:250–255. https://doi.org/10.1016/j.bbr.2017.09.008
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504. https://doi.org/10.1037/0033-2909.83.3.482
Loetchutinat C, Kothan S, Dechsupa S et al (2005) Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Radiat Phys Chem 72:323–331. https://doi.org/10.1016/j.radphyschem.2004.06.011
Miranda KM, Espey MG, Yamada K et al (2001) Unique oxidative mechanisms for the reactive nitrogen oxide species, nitroxyl anion. J Biol Chem 276:1720–1727. https://doi.org/10.1074/JBC.M006174200
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–33. https://doi.org/10.1016/s0076-6879(81)77046-0
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Fc T, Jm G, Msp S et al (2020) Inosine protects against impairment of memory induced by experimental model of Alzheimer disease: a nucleoside with multitarget brain actions. Psychopharmacology 237:811–823. https://doi.org/10.1007/S00213-019-05419-5
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/ABIO.1976.9999
Curtis MJ, Alexander S, Cirino G et al (2018) Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175:987–993. https://doi.org/10.1111/bph.14153
Starobova H, Vetter I (2017) Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci 10:1–21. https://doi.org/10.3389/fnmol.2017.00174
Colloca L, Ludman T, Bouhassira D et al (2017) Neuropathic pain [Review]. Nat Rev Dis Prim 3:17002. https://doi.org/10.1038/nrdp.2017.2
Cruz-Almeida Y, Fillingim RB, Riley JL et al (2019) Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain 160:1119–1130. https://doi.org/10.1097/j.pain.0000000000001491
Cruz-Almeida Y, Cole J (2020) Pain, aging, and the brain: new pieces to a complex puzzle. Pain 161:461–463
Zhang H, Davies KJA, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88:314–336
Korovila I, Hugo M, Castro JP, Weber D, Höhn A, Grune T, Jung T (2017) Proteostasis, oxidative stress and aging. Redox Biol 13:550–567. https://doi.org/10.1016/j.redox.2017.07.008
Nozadze E, Arutinova N, Tsakadze L et al (2015) Molecular mechanism of Mg-ATPase activity. J Membr Biol 248:295–300. https://doi.org/10.1007/s00232-014-9769-2
Wang XJ, Li Y, Luo L et al (2014) Oxaliplatin activates the Keap1/Nrf2 antioxidant system conferring protection against the cytotoxicity of anticancer drugs. Free Radic Biol Med 70:68–77. https://doi.org/10.1016/j.freeradbiomed.2014.02.010
Ge Y, Jiao Y, Li P, Xiang Z, Li Z, Wang L, Li W, Gao H, Shao J, Wen D, Weifeng Y (2018) Coregulation of endoplasmic reticulum stress and oxidative stress in neuropathic pain and disinhibition of the spinal nociceptive circuitry. Pain 159(5):894–906. https://doi.org/10.1097/j.pain.0000000000001161
Khasabova IA, Khasabov SG, Olson JK et al (2019) Pioglitazone, a PPARγ agonist, reduces cisplatin-evoked neuropathic pain by protecting against oxidative stress. Pain 160:688–701. https://doi.org/10.1097/j.pain.0000000000001448
Huličiak M, Vacek J, Šebela M et al (2012) Covalent binding of cisplatin impairs the function of Na +/K +-ATPase by binding to its cytoplasmic part. Biochem Pharmacol 83:1507–1513. https://doi.org/10.1016/j.bcp.2012.02.015
Kubala M, Geleticova J, Huliciak M et al (2014) Na+/K+-ATPase inhibition by cisplatin and consequences for cisplatin nephrotoxicity. Biomed Pap 158:194–200. https://doi.org/10.5507/bp.2014.018
Tummala R, Wolle D, Barwe SP et al (2009) Expression of Na, K-ATPase-β1 subunit increases uptake and sensitizes carcinoma cells to oxaliplatin. Cancer Chemother Pharmacol 64:1187–1194. https://doi.org/10.1007/s00280-009-0985-x
Areti A, Yerra VG, Naidu VGM, Kumar A (2014) Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol 2:289–295. https://doi.org/10.1016/j.redox.2014.01.006
Kanat O, Ertas H, Caner B (2017) Platinum-induced neurotoxicity: a review of possible mechanisms. World J Clin Oncol 8:329–333. https://doi.org/10.5306/wjco.v8.i4.329
Munhoz CD, Kawamoto EM, De Sá LL et al (2005) Glutamate modulates sodium-potassium-ATPase through cyclic GMP and cyclic GMP-dependent protein kinase in rat striatum. Cell Biochem Funct 23:115–123. https://doi.org/10.1002/cbf.1217
Reis AS, Pinz M, Duarte LFB et al (2017) 4-phenylselenyl-7-chloroquinoline, a novel multitarget compound with anxiolytic activity: contribution of the glutamatergic system. J Psychiatr Res 84:191–199. https://doi.org/10.1016/j.jpsychires.2016.10.007
Silva VDG, Reis AS, Pinz MP et al (2017) Further analysis of acute antinociceptive and anti-inflammatory actions of 4-phenylselenyl-7-chloroquinoline in mice. Fundam Clin Pharmacol 31:513–525. https://doi.org/10.1111/fcp.12295
Funding
This study received financial support and scholarships from the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (429859/2018–0, 312747/2020–9), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) (PqG 17/2551–0001013-2). This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível superior – Brasil (CAPES)—Finance Code 001. C.L.; E.A.W.; D.A. are recipients of CNPq fellowship. This study also received financial assistance from L’ORÉAL-UNESCO-ABC for Women in Science.
Author information
Authors and Affiliations
Contributions
A.S.R., C.C.M., K.P.M, J.J.P., C.L., and E.A.W. conceived and designed the study. G.P.C. and D.A. performed the 4-PSQ synthesis. A.S.R. and E.A.W. wrote the manuscript. E.A.W. supervised the study. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Animal care and all experimental procedures were conducted in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publications no. 80–23, revised in 1996). Also, this study was performed in line with the principles of the Declaration of Helsinki and in accordance with the Committee on Care and Use of Experimental Animal Resources, Federal University of Pelotas, Brazil (CEEA 4506–2017). All efforts were made to minimize the number of animals used and their suffering.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reis, A.S., Martins, C.C., da Motta, K.P. et al. Interface of Aging and Acute Peripheral Neuropathy Induced by Oxaliplatin in Mice: Target-Directed Approaches for Na+, K+—ATPase, Oxidative Stress, and 7-Chloro-4-(phenylselanyl) quinoline Therapy. Mol Neurobiol 59, 1766–1780 (2022). https://doi.org/10.1007/s12035-021-02659-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02659-5