Abstract
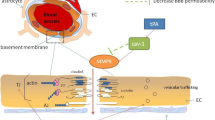
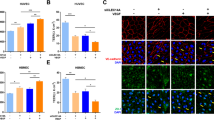
Thrombolytic therapy with recombinant tissue plasminogen activator (rt-PA) is currently the only FDA-approved drug for acute ischemic stroke. However, its administration is still limited due to the associated increased risk of hemorrhagic transformation (HT). rt-PA may exacerbate blood-brain barrier (BBB) injury by several mechanisms that have not been fully elucidated. Caveolin-1 (Cav-1), a major structural protein of caveolae, has been linked to the endothelial barrier function. The effects of rt-PA on Cav-1 expression remain largely unknown. Here, Cav-1 protein expression after ischemic conditions, with or without rt-PA administration, was analyzed in a murine thromboembolic middle cerebral artery occlusion (MCAO) and in brain microvascular endothelial bEnd.3 cells subjected to oxygen/glucose deprivation (OGD). Our results show that Cav-1 is overexpressed in endothelial cells of infarcted area and in bEnd.3 cell line after ischemia but there is disagreement regarding rt-PA effects on Cav-1 expression between both experimental models. Delayed rt-PA administration significantly reduced Cav-1 total levels from 24 to 72 h after reoxygenation and increased pCav-1/Cav-1 at 72 h in the bEnd.3 cells while it did not modify Cav-1 immunoreactivity in the infarcted area at 24 h post-MCAO. Importantly, tissue Cav-1 positively correlated with Cav-1 serum levels at 24 h post-MCAO and negatively correlated with the volume of hemorrhage after infarction, the latter supporting a protective role of Cav-1 in cerebral ischemia. In addition, the negative association between baseline serum Cav-1 levels and hemorrhagic volume points to a potential usefulness of baseline serum Cav-1 levels to predict hemorrhagic volume, independently of rt-PA administration.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BBB:
-
Blood-brain barrier
- bEnd.3:
-
Immortalized mouse brain endothelial cell line
- BSA:
-
Bovine serum albumin
- Cav-1:
-
Caveolin-1
- CTR:
-
Control
- DAB:
-
Diaminobenzidine
- FITC-BSA:
-
Fluorescein isothiocyanate labelled bovine serum albumin
- HT:
-
Hemorrhagic transformation
- MCAO:
-
Middle cerebral artery occlusion
- MTT:
-
3-(4,5-Dimethylthiazol)-2,5-diphenyl tetrazolium bromide
- OGD:
-
Oxygen/glucose deprivation
- pCav-1:
-
Phosphorylated caveolin-1
- rt-PA:
-
Recombinant tissue plasminogen activator
References
Kim J, Thayabaranathan T, Donnan GA et al (2020) Global Stroke Statistics 2019. Int J Stroke 15:819–838. https://doi.org/10.1177/1747493020909545
Pandian JD, Gall SL, Kate MP et al (2018) Prevention of stroke: a global perspective. Lancet 392:1269–1278. https://doi.org/10.1016/S0140-6736(18)31269-8
Fusco R, Scuto M, Cordaro M et al (2019) N-palmitoylethanolamide-oxazoline protects against middle cerebral artery occlusion injury in diabetic rats by regulating the SIRT1 pathway. Int J Mol Sci 20:1–22. https://doi.org/10.3390/ijms20194845
Powers WJ, Rabinstein AA, Ackerson T et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke A
Aguiar de Sousa D, von Martial R, Abilleira S et al (2019) Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J 4:13–28. https://doi.org/10.1177/2396987318786023
Liu C, Xie J, Sun S et al (2020) Hemorrhagic transformation after tissue plasminogen activator treatment in acute ischemic stroke. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-020-00985-1
Fanne RA, Nassar T, Yarovoi S et al (2010) Blood-brain barrier permeability and tPA-mediated neurotoxicity. Neuropharmacology 58:972–980. https://doi.org/10.1016/j.neuropharm.2009.12.017
Won SJ, Tang XN, Suh SW et al (2011) Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by increasing superoxide production. Ann Neurol 70:583–590. https://doi.org/10.1002/ana.22538
Adibhatla R, Hatcher J (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord - Drug Targets 7:243–253. https://doi.org/10.2174/187152708784936608
Zhao YL, Song JN, Zhang M (2014) Role of caveolin-1 in the biology of the blood-brain barrier. Rev Neurosci 25:247–254. https://doi.org/10.1515/revneuro-2013-0039
Zhou M, Shi SX, Liu N et al (2021) Caveolae-mediated endothelial transcytosis across the blood-brain barrier in acute ischemic stroke. J Clin Med. https://doi.org/10.3390/jcm10173795
Gu Y, Zheng G, Xu M et al (2012) Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J Neurochem 120:147–156. https://doi.org/10.1111/j.1471-4159.2011.07542.x
Jasmin J-FJF, Malhotra S, Singh Dhallu M et al (2007) Caveolin-1 deficiency increases cerebral ischemic injury. Circ Res 100:721–729. https://doi.org/10.1161/01.RES.0000260180.42709.29
Nag S, Venugopalan R, Stewart DJ (2007) Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown. Acta Neuropathol 114:459–469. https://doi.org/10.1007/s00401-007-0274-x
Song H, Cheng Y, Bi G et al (2016) Release of matrix metalloproteinases-2 and 9 by S-nitrosylated caveolin-1 contributes to degradation of extracellular matrix in tPA-treated hypoxic endothelial cells. PLoS ONE 11:1–16. https://doi.org/10.1371/journal.pone.0149269
Nag S, Manias JLL, Stewart DJJ (2009) Expression of endothelial phosphorylated caveolin-1 is increased in brain injury. Neuropathol Appl Neurobiol 35:417–426. https://doi.org/10.1111/j.1365-2990.2008.01009.x
García-Yébenes I, Sobrado M, Zarruk JGG et al (2011) A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke 42:196–203. https://doi.org/10.1161/STROKEAHA.110.600452
Cyrille O, Audrey LB, Anne-Laure B et al (2007) Mouse model of in situ thromboembolic stroke and reperfusion. Stroke 38:2771–2778. https://doi.org/10.1007/978-1-4939-5620-3_6
Arnberg F, Grafström J, Lundberg J et al (2015) Imaging of a clinically relevant stroke model glucose hypermetabolism revisited. Stroke 46:835–842. https://doi.org/10.1161/STROKEAHA.114.008407
Gubern C, Comajoan P, Huguet G et al (2020) Evaluation of long-term rt-PA effects on bEnd.3 endothelial cells under ischemic conditions; changes in ZO-1 expression and glycosylation of the bradykinin B2 receptor. Thromb Res 187. https://doi.org/10.1016/j.thromres.2019.12.021
Brown RC, Morris AP, O’Neil RG (2007) Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res 1130:17–30. https://doi.org/10.1016/j.brainres.2006.10.083
Comajoan P, Gubern C, Huguet G et al (2018) Evaluation of common housekeeping proteins under ischemic conditions and/or rt-PA treatment in bEnd.3 cells. J Proteomics 184. https://doi.org/10.1016/j.jprot.2018.06.011
Godfrey KRKR, Tanswell P, Bates RAR et al (1998) Nonlinear pharmacokinetics of tissue-type plasminogen activator in three animal species: a comparison of mathematical models. Biopharm Drug Dispos 19:131–140. https://doi.org/10.1002/(SICI)1099-081X(199803)19:2%3c131::AID-BDD87%3e3.0.CO;2-L
Su EJ, Fredriksson L, Geyer M et al (2008) Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med 14:731–737. https://doi.org/10.1038/nm1787
Choi KH, Kim HS, Park MS et al (2016) Regulation of Caveolin-1 expression determines early brain edema after experimental focal cerebral ischemia. Stroke 47:1336–1343. https://doi.org/10.1161/STROKEAHA.116.013205
Chen S, Chen Z, Cui J et al (2018) Early abrogation of gelatinase activity extends the time window for tpa thrombolysis after embolic focal cerebral ischemia in mice. eNeuro 5. https://doi.org/10.1523/ENEURO.0391-17.2018
Nag S (2003) Pathophysiology of blood-brain barrier breakdown. Methods Mol Med 89:97–119. https://doi.org/10.1385/1-59259-419-0:97
Blochet C, Buscemi L, Clément T et al (2020) Involvement of caveolin-1 in neurovascular unit remodeling after stroke: effects on neovascularization and astrogliosis. J Cereb Blood Flow Metab 40:163–176. https://doi.org/10.1177/0271678X18806893
Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG (2020) Metalloproteinases and their inhibitors: potential for the development of new therapeutics. Cells 9:1–34. https://doi.org/10.3390/cells9051313
Castellanos M, Van Eendenburg C, Gubern C et al (2018) Low levels of caveolin-1 predict symptomatic bleeding after thrombolytic therapy in patients with acute ischemic stroke. Stroke 49:1525–1527. https://doi.org/10.1161/STROKEAHA.118.020683
Zhang J, Zhu W, Xiao L et al (2016) Lower serum Caveolin-1 is associated with cerebral microbleeds in patients with acute ischemic stroke. Oxid Med Cell Longev. https://doi.org/10.1155/2016/9026787
Watanabe T, Dohgu S, Takata F et al (2013) Paracellular barrier and tight junction protein expression in the immortalized brain endothelial cell lines bend.3, bend.5 and mouse brain endothelial cell 4. Biol Pharm Bull 36:492–495. https://doi.org/10.1248/bpb.b12-00915
Liu J, Jin X, Liu KJKJ, Liu W (2012) Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 32:3044–3057. https://doi.org/10.1523/JNEUROSCI.6409-11.2012
Yang MC, Zhang HZ, Wang Z et al (2016) The molecular mechanism and effect of cannabinoid-2 receptor agonist on the blood-spinal cord barrier permeability induced by ischemia-reperfusion injury. Brain Res 1636:81–92. https://doi.org/10.1016/j.brainres.2016.01.047
Lee H, Volonte’ D, Galbiati F et al (2000) Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: Identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol 14:1750–1775. https://doi.org/10.1210/mend.14.11.0553
Li S, Seitz R, Lisanti MP (1996) Phosphorylation of caveolin by Src tyrosine kinases: the α-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem 271:3863–3868. https://doi.org/10.1074/jbc.271.7.3863
Zimnicka AMAM, Husain YSYS, Shajahan ANAN et al (2016) Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Mol Biol Cell 27:2090–2106. https://doi.org/10.1091/mbc.E15-11-0756
Sun Y, Hu G, Zhang X, Minshall RDD (2009) Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res 105:676–685. https://doi.org/10.1161/CIRCRESAHA.109.201673
Coelho-Santos V, Socodato R, Portugal C et al (2016) Methylphenidate-triggered ROS generation promotes caveolae-mediated transcytosis via Rac1 signaling and c-Src-dependent caveolin-1 phosphorylation in human brain endothelial cells. Cell Mol Life Sci 73:4701–4716. https://doi.org/10.1007/s00018-016-2301-3
Rui Q, Ni H, Lin X, et al (2019) Astrocyte-derived fatty acid-binding protein 7 protects blood-brain barrier integrity through a caveolin-1/MMP signaling pathway following traumatic brain injury. Exp Neurol 322. https://doi.org/10.1016/j.expneurol.2019.113044
Acknowledgements
The authors thank Dr. Maria Buxó for her support in the statistical data analysis.
Funding
This work was supported by grants from Instituto de Salud Carlos III and co-financed by the European Development Regional Fund “A Way to Achieve Europe” Health Strategic Action Program PI13/02258 and PI17/02123 (MC), PI20/00535 (IL), and Spanish Stroke Research Network RETICS RD12/0014/0010 (MC), and RD16/0019/0003 (JS), RD16/0019/0004 (MC), and RD16/0019/0009 (IL); from Regional Madrid Government B2017/BMD- 3688 (IL); from Spanish Ministry of Science and Innovation PID2019-106581RB-I00 (MAM); from Leducq Foundation for Cardiovascular Research TNE-19CVD01 (MAM); and from Fundación La Caixa HR17_00527 (MAM). P. Comajoan was a recipient of a predoctoral fellowship from the University of Girona (IF-UdG 2015).
Author information
Authors and Affiliations
Author notes
Carme Gubern-Mérida and Pau Comajoan are joint first authors.
Elisabet Kádár and Mar Castellanos are joint last authors.
Contributions
CG, PC, and EK performed in vitro procedures and Western blot, ELISA, and immunofluorescence analysis. IP helped with immunohistochemistry analysis. IG performed the mouse thromboembolic stroke model. CG, PC, EK, and GH designed the experiments and interpreted data. IL, MAM, JS, and MC contributed to data interpretation and, joint to JMS, critically revised the manuscript. CG, PC, and EK were the major contributors in writing the manuscript. All the authors read and approved the final manuscript and agreed to be accountable in ensuring appropriate answer to questions related to the accuracy and integrity of any part of the work.
Corresponding authors
Ethics declarations
Ethics Approval
All procedures were performed in accordance with the European Communities Council Directive (86/609/EEC) and approved by the Ethics Committee on Animal Welfare of University Complutense (PROEX No. 016/18) and are reported according to ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Consent to Participate
Not applicable
Consent to Publish
All the authors verify that they concur with the present submission and that the material submitted has not been previously reported in any other journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gubern-Mérida, C., Comajoan, P., Huguet, G. et al. Cav-1 Protein Levels in Serum and Infarcted Brain Correlate with Hemorrhagic Volume in a Mouse Model of Thromboembolic Stroke, Independently of rt-PA Administration. Mol Neurobiol 59, 1320–1332 (2022). https://doi.org/10.1007/s12035-021-02644-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02644-y