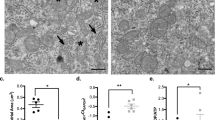
Abstract
Anesthetics are commonly used in various medical procedures. Accumulating evidence suggests that early-life anesthetics exposure in infants and children affects brain development, causing psychiatric and neurological disorders. However, the underlying mechanisms are poorly understood. Using zebrafish larvae as a model, we found that the proliferation and migration of oligodendrocyte progenitor cells (OPCs) were severely impaired by the exposure of midazolam (MDZ), an anesthetic widely used in pediatric surgery and intensive care medicine, leading to a reduction of oligodendroglial lineage cell in the dorsal spinal cord. This defect was mimicked by the bath application of translocator protein (TSPO) agonists and partially rescued by genetic downregulation of TSPO. Cell transplantation experiments showed that requirement of TSPO for MDZ-induced oligodendroglial lineage cell defects is cell-autonomous. Furthermore, transmission electron microscopy and in vivo electrophysiological recording experiments demonstrated that MDZ exposure caused axon hypomyelination and action potential propagation retardation, resulting in delayed behavior initiation. Thus, our findings reveal that MDZ affects oligodendroglial lineage cell development and myelination in young animals, raising the care about its clinic use in infants and children.






Similar content being viewed by others
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Raper J, De Biasio JC, Murphy KL, Alvarado MC, Baxter MG (2018) Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth 120(4):761–767. https://doi.org/10.1016/j.bja.2018.01.014
Kang E, Jiang D, Ryu YK, Lim S, Kwak M, Gray CD, Xu M, Choi JH, Junn S, Kim J, Xu J, Schaefer M, Johns RA, Song H, Ming GL, Mintz CD (2017) Early postnatal exposure to isoflurane causes cognitive deficits and disrupts development of newborn hippocampal neurons via activation of the mTOR pathway. PLoS Biol 15(7):e2001246. https://doi.org/10.1371/journal.pbio.2001246
Wang Q, Shen FY, Zou R, Zheng JJ, Yu X, Wang YW (2017) Ketamine-induced apoptosis in the mouse cerebral cortex follows similar characteristic of physiological apoptosis and can be regulated by neuronal activity. Mol Brain 10(1):24. https://doi.org/10.1186/s13041-017-0302-2
Diana P, Joksimovic SM, Faisant A, Jevtovic-Todorovic V (2019) Early exposure to general anesthesia impairs social and emotional development in rats. Mol Neurobiol. https://doi.org/10.1007/s12035-019-01755-x
Zaccariello MJ, Frank RD, Lee M, Kirsch AC, Schroeder DR, Hanson AC, Schulte PJ, Wilder RT, Sprung J, Katusic SK, Flick RP, Warner DO (2019) Patterns of neuropsychological changes after general anaesthesia in young children: secondary analysis of the Mayo Anesthesia Safety in Kids study. Br J Anaesth 122(5):671–681. https://doi.org/10.1016/j.bja.2019.01.022
Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F (2017) Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr 171(1):e163470. https://doi.org/10.1001/jamapediatrics.2016.3470
Walkden GJ, Gill H, Davies NM, Peters AE, Wright I, Pickering AE (2020) Early childhood general anesthesia and neurodevelopmental outcomes in the Avon Longitudinal Study of Parents and Children birth cohort. Anesthesiology 133(5):1007–1020. https://doi.org/10.1097/ALN.0000000000003522
Polaner DM, Zuk J, McCann ME, Davidson A (2017) Warnings, uncertainty, and clinical practice. Lancet 389(10085):2174–2176. https://doi.org/10.1016/S0140-6736(17)31506-4
Loepke AW (2010) Developmental neurotoxicity of sedatives and anesthetics: a concern for neonatal and pediatric critical care medicine? Pediatr Crit Care Med 11(2):217–226. https://doi.org/10.1097/PCC.0b013e3181b80383
Ng E, Taddio A, Ohlsson A (2017) Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev 1:CD002052. https://doi.org/10.1002/14651858.CD002052.pub3
Vutskits L, Xie Z (2016) Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci 17(11):705–717. https://doi.org/10.1038/nrn.2016.128
Suminaite D, Lyons DA, Livesey MR (2019) Myelinated axon physiology and regulation of neural circuit function. Glia 67(11):2050–2062. https://doi.org/10.1002/glia.23665
Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487(7408):443–448. https://doi.org/10.1038/nature11314
Gelabert-Gonzalez M, Ginesta-Galan V, Sernamito-Garcia R, Allut AG, Bandin-Dieguez J, Rumbo RM (2006) The Camino intracranial pressure device in clinical practice. Assessment in a 1000 cases. Acta Neurochir (Wien) 148(4):435–441. https://doi.org/10.1007/s00701-005-0683-3
Yeung MSY, Djelloul M, Steiner E, Bernard S, Salehpour M, Possnert G, Brundin L, Frisen J (2019) Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566(7745):538–542. https://doi.org/10.1038/s41586-018-0842-3
Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, Du X, Sampath H, Bruce H, Chiappelli J, Ryan M, Fisseha F, Savransky A, Adhikari B, Chen S, Paciga SA, Whelan CD, Xie Z, Hyde CL, Chen X, Schubert CR, O’Donnell P, Hong LE (2017) Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiat 74(9):958–966. https://doi.org/10.1001/jamapsychiatry.2017.2228
Banerjee P, Rossi MG, Anghelescu DL, Liu W, Breazeale AM, Reddick WE, Glass JO, Phillips NS, Jacola LM, Sabin ND, Inaba H, Srivastava D, Robison LL, Pui CH, Hudson MM, Krull KR (2019) Association between anesthesia exposure and neurocognitive and neuroimaging outcomes in long-term survivors of childhood acute lymphoblastic leukemia. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.1094
Paquet D, Bhat R, Sydow A, Mandelkow EM, Berg S, Hellberg S, Falting J, Distel M, Koster RW, Schmid B, Haass C (2009) A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J Clin Invest 119(5):1382–1395. https://doi.org/10.1172/JCI37537
Xu DJ, Bu JW, Gu SY, Xia YM, Du JL, Wang YW (2011) Celecoxib impairs heart development via inhibiting cyclooxygenase-2 activity in zebrafish embryos. Anesthesiology 114(2):391–400. https://doi.org/10.1097/ALN.0b013e3182039f22
Xu DJ, Wang B, Zhao X, Zheng Y, Du JL, Wang YW (2017) General anesthetics protects against cardiac arrest-induced brain injury by inhibiting calcium wave propagation in zebrafish. Mol Brain 10(1):44. https://doi.org/10.1186/s13041-017-0323-x
Shin J, Park HC, Topczewska JM, Mawdsley DJ, Appel B (2003) Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci 25(1–2):7–14. https://doi.org/10.1023/B:MICS.0000006847.09037.3a5146566
Lee JS, Padmanabhan A, Shin J, Zhu S, Guo F, Kanki JP, Epstein JA, Look AT (2010) Oligodendrocyte progenitor cell numbers and migration are regulated by the zebrafish orthologs of the NF1 tumor suppressor gene. Hum Mol Genet 19(23):4643–4653. https://doi.org/10.1093/hmg/ddq395ddq395
Rampon C, Bouzaffour M, Ostuni MA, Dufourcq P, Girard C, Freyssinet JM, Lacapere JJ, Schweizer-Groyer G, Vriz S (2009) Translocator protein (18 kDa) is involved in primitive erythropoiesis in zebrafish. FASEB J 23(12):4181–4192. https://doi.org/10.1096/fj.09-129262fj.09-129262
Chen Q, Jiang L, Li C, Hu D, Bu JW, Cai D, Du JL (2012) Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS Biol 10(8):e1001374. https://doi.org/10.1371/journal.pbio.1001374
Xu Q (1999) Microinjection into zebrafish embryos. Methods Mol Biol 127:125–132. https://doi.org/10.1385/1-59259-678-9:125
Liu P, Du JL, He C (2013) Developmental pruning of early-stage myelin segments during CNS myelination in vivo. Cell Res 23(7):962–964. https://doi.org/10.1038/cr.2013.62
Yu PC, Gu SY, Bu JW, Du JL (2010) TRPC1 is essential for in vivo angiogenesis in zebrafish. Circ Res 106(7):1221–1232. https://doi.org/10.1161/CIRCRESAHA.109.207670
Kimmel CB, Warga RM, Schilling TF (1990) Origin and organization of the zebrafish fate map. Development 108(4):581–594
Mu Y, Li XQ, Zhang B, Du JL (2012) Visual input modulates audiomotor function via hypothalamic dopaminergic neurons through a cooperative mechanism. Neuron 75(4):688–699. https://doi.org/10.1016/j.neuron.2012.05.035
Czopka T, Ffrench-Constant C, Lyons DA (2013) Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell 25(6):599–609. https://doi.org/10.1016/j.devcel.2013.05.013
Nicolay DJ, Doucette JR, Nazarali AJ (2007) Transcriptional control of oligodendrogenesis. Glia 55(13):1287–1299. https://doi.org/10.1002/glia.20540
Tokuda K, O’Dell KA, Izumi Y, Zorumski CF (2010) Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J Neurosci 30(50):16788–16795. https://doi.org/10.1523/JNEUROSCI.4101-10.201030/50/16788
Barron AM, Higuchi M, Hattori S, Kito S, Suhara T, Ji B (2021) Regulation of anxiety and depression by mitochondrial translocator protein-mediated steroidogenesis: the role of neurons. Mol Neurobiol 58(2):550–563. https://doi.org/10.1007/s12035-020-02136-5
Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B (2006) In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci 9(12):1506–1511. https://doi.org/10.1038/nn1803
Zhou B, Chen L, Liao P, Huang L, Chen Z, Liao D, Yang L, Wang J, Yu G, Wang L, Zhang J, Zuo Y, Liu J, Jiang R (2019) Astroglial dysfunctions drive aberrant synaptogenesis and social behavioral deficits in mice with neonatal exposure to lengthy general anesthesia. PLoS Biol 17(8):e3000086. https://doi.org/10.1371/journal.pbio.3000086
Li Q, Mathena RP, Xu J, Eregha ON, Wen J, Mintz CD (2019) Early postnatal exposure to isoflurane disrupts oligodendrocyte development and myelin formation in the mouse hippocampus. Anesthesiology. https://doi.org/10.1097/ALN.0000000000002904
Ohta N, Ohashi Y, Takayama C, Mashimo T, Fujino Y (2011) Midazolam suppresses maturation of murine dendritic cells and priming of lipopolysaccharide-induced t helper 1-type immune response. Anesthesiology 114(2):355–362. https://doi.org/10.1097/ALN.0b013e3182070c1f
Tanabe K, Kozawa O, Iida H (2011) Midazolam suppresses interleukin-1beta-induced interleukin-6 release from rat glial cells. J Neuroinflammation 8:68. https://doi.org/10.1186/1742-2094-8-68
Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW (2005) Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol 146(2):189–197. https://doi.org/10.1038/sj.bjp.0706301
Joo HK, Lee YR, Kang G, Choi S, Kim CS, Ryoo S, Park JB, Jeon BH (2015) The 18-kDa translocator protein inhibits vascular cell adhesion molecule-1 expression via inhibition of mitochondrial reactive oxygen species. Mol Cells 38(12):1064–1070. https://doi.org/10.14348/molcells.2015.0165
Stevens MF, Werdehausen R, Gaza N, Hermanns H, Kremer D, Bauer I, Kury P, Hollmann MW, Braun S (2011) Midazolam activates the intrinsic pathway of apoptosis independent of benzodiazepine and death receptor signaling. Reg Anesth Pain Med 36(4):343–349. https://doi.org/10.1097/AAP.0b013e318217a6c7
So EC, Chang YT, Hsing CH, Poon PW, Leu SF, Huang BM (2010) The effect of midazolam on mouse Leydig cell steroidogenesis and apoptosis. Toxicol Lett 192(2):169–178. https://doi.org/10.1016/j.toxlet.2009.10.017
Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M (2010) Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 9(12):971–988. https://doi.org/10.1038/nrd3295nrd3295
Guilarte TR (2019) TSPO in diverse CNS pathologies and psychiatric disease: a critical review and a way forward. Pharmacol Ther 194:44–58. https://doi.org/10.1016/j.pharmthera.2018.09.003
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31(7):361–370. https://doi.org/10.1016/j.tins.2008.04.001S0166-2236(08)00132-X
Alnaes D, Kaufmann T, Doan NT, Cordova-Palomera A, Wang Y, Bettella F, Moberget T, Andreassen OA, Westlye LT (2018) Association of heritable cognitive ability and psychopathology with white matter properties in children and adolescents. JAMA Psychiat 75(3):287–295. https://doi.org/10.1001/jamapsychiatry.2017.4277
Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO (2012) Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 87(2):120–129. https://doi.org/10.1016/j.mayocp.2011.11.008S0025-6196(11)00072-3
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Hu D, Voigt RG, Paule MG, Chelonis JJ, Flick RP (2018) Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology 129(1):89–105. https://doi.org/10.1097/ALN.0000000000002232
Clausen NG, Kahler S, Hansen TG (2018) Systematic review of the neurocognitive outcomes used in studies of paediatric anaesthesia neurotoxicity. Br J Anaesth 120(6):1255–1273. https://doi.org/10.1016/j.bja.2017.11.107
McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, Grobler A, Stargatt R, Hunt RW, Sheppard SJ, Marmor J, Giribaldi G, Bellinger DC, Hartmann PL, Hardy P, Frawley G, Izzo F, von Ungern Sternberg BS, Lynn A, Wilton N, Mueller M, Polaner DM, Absalom AR, Szmuk P, Morton N, Berde C, Soriano S, Davidson AJ, Consortium GAS (2019) Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393(10172):664–677. https://doi.org/10.1016/S0140-6736(18)32485-1
Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR, DiMaggio CJ, Cooper TJ, Rauh V, Maxwell LG, Youn A, McGowan FX (2016) Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 315(21):2312–2320. https://doi.org/10.1001/jama.2016.6967
Xu J, Mathena RP, Singh S, Kim J, Long JJ, Li Q, Junn S, Blaize E, Mintz CD (2019) Early developmental exposure to repetitive long duration of midazolam sedation causes behavioral and synaptic alterations in a rodent model of neurodevelopment. J Neurosurg Anesthesiol 31(1):151–162. https://doi.org/10.1097/ANA.0000000000000541
Alvarez RV, Palmer C, Czaja AS, Peyton C, Silver G, Traube C, Mourani PM, Kaufman J (2018) Delirium is a common and early finding in patients in the pediatric cardiac intensive care unit. J Pediatr 195:206–212. https://doi.org/10.1016/j.jpeds.2017.11.064
Mulla H, Lawson G, Peek GJ, Firmin RK, Upton DR (2003) Plasma concentrations of midazolam in neonates receiving extracorporeal membrane oxygenation. ASAIO J 49(1):41–47. https://doi.org/10.1097/00002480-200301000-00007
Fukuoka N, Aibiki M, Tsukamoto T, Seki K, Morita S (2004) Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation 60(2):225–230. https://doi.org/10.1016/j.resuscitation.2003.09.017
Acknowledgements
We thank Dr Bruce Appel at University of Colorado School of Medicine for providing the Tg(olig2:EGFP) transgenic zebrafish, Sox10:mRFP plasmid and anti-sox10 antibody.
Funding
This work was supported by the National Natural Science Foundation of China (grants no. 81200942, 81671058 and 81730031) and the Foundation of Shanghai Municipal Key Clinical Specialty (grant no. shslczdzk06901).
Author information
Authors and Affiliations
Contributions
Daojie Xu designed, planned and performed the experiments, analyzed the data, and wrote the manuscript. Bin Wang performed DNA microinjection and assisted with cell transplantation experiments. Bo Xu performed immunohistochemistry and assisted with time-lapse imaging experiments. Chen Yin performed in vivo electrophysiological recording. Li Ning assisted with transmission electron microscopy and drug treatment experiments. Xiaoquan Li assisted with C-start escape behavior test. Jiulin Du oversaw all research phases and contributed to writing the manuscript. Yingwei Wang coordinated and supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethical Statement
All animal work were approved by Institute of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (AVI 1203 KB)
Supplementary file3 (AVI 1028 KB)
Rights and permissions
About this article
Cite this article
Xu, D., Wang, B., Xu, B. et al. Midazolam Exposure Impedes Oligodendrocyte Development via the Translocator Protein and Impairs Myelination in Larval Zebrafish. Mol Neurobiol 59, 93–106 (2022). https://doi.org/10.1007/s12035-021-02559-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02559-8