Abstract
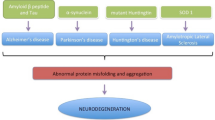
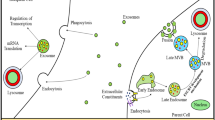
Alzheimer’s disease (AD) is the most common neurodegenerative disease. It is known to be a multifactorial disease and several causes are associated with its occurrence as well as progression. However, the accumulation of amyloid beta (Aβ) is widely considered its major pathogenic hallmark. Additionally, neurofibrillary tangles (NFT), mitochondrial dysfunction, oxidative stress, and aging (cellular senescence) are considered as additional hits affecting the disease pathology. Several studies are now suggesting important role of inflammation in AD, which shifts our thought towards the brain’s resident immune cells, microglia, and astrocytes; how they interact with neurons; and how these interactions are affected by intra and extracellular stressful factors. These interactions can be modulated by different mechanisms and pathways, in which exosomes could play an important role. Exosomes are multivesicular bodies secreted by nearly all types of cells. The exosomes secreted by glial cells or neurons affect the interactions and thus the physiology of these cells by transmitting miRNAs, proteins, and lipids. Exosomes can serve as a friend or foe to the neuron function, depending upon the carried signals. Exosomes, from the healthy microenvironment, may assist neuron function and health, whereas, from the stressed microenvironment, they carry oxidative and inflammatory signals to the neurons and thus prove detrimental to the neuronal function. Furthermore, exosomes can cross the blood–brain barrier (BBB), and from the blood plasma they can enter the brain cells and activate microglia and astrocytes. Exosomes can transport Aβ or Tau, cytokines, miRNAs between the cells, and alter the physiology of recipient cells. They can also assist in Aβ clearance and regulation of synaptic activity. The exosomes derived from different cells play different roles, and this field is still in its infancy stage. This review advocates exosomes’ role as a friend or foe in neurodegenerative diseases, especially in the case of Alzheimer’s disease.





Similar content being viewed by others
Data Availability
As this is a review article, relevant information collected and presented from the public domain available to the scientific community.
References
Lee S, Mankhong S, Kang JH (2019) Extracellular vesicle as a source of Alzheimer’s biomarkers: opportunities and challenges. Int J Mol Sci 20:1728
Malm T, Loppi S, Kanninen KM (2018) Exosomes in Alzheimer’s disease. Neurochem Int 97:193–199
Al-Nedawi K (2014) The Yin-Yang of microvesicles (exosomes) in cancer biology. Front Oncol 4:172
Vlassov AV, Magdaleno S, Setterquist R, Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820:940–948
Beach A, Zhang HG, Ratajczak MZ, Kakar SS (2014) Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res 7:1–11
Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci 91:4766–4770
Gao G, Zhao S, Xia X, Li C, Li C, Ji C, Sheng S, Tang Y et al (2019) Glutaminase C regulates microglial activation and pro-inflammatory exosome release, relevance to the pathogenesis of Alzheimer’s disease. Front Cell Neurosci 13:264
Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Brigstock DR (2014) Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 59(3):1118–1129
Schneider A, Simons M (2013) Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res 352(1):33–47
Yuyama K, Mitsutake S, Igarashi Y (2014A) Pathological Roles of ceramide and its metabolites in metabolic syndrome and Alzheimer’s disease. Biochim Biophys Acta 1841:793–798
Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa J, Fujitani N et al (2014B) Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem 289(35):24488–24498
Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Goetzl EJ (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement 11(6):600–607
Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Miller BL (2016) Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J 30(11):3853–3859
Shi M, Kovac A, Korff A, Cook TJ, Ginghina C, Bullock KM, Zhang J (2016) CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement 12(11):1125–1131
Candelario KM, Steindler DA (2014) The role of extracellular vesicles in the progression of neurodegenerative disease and cancer. Trends Mol Med 20(7):368–374
Kalani A, Tyagi A, Tyagi N (2014) Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol 49:590–600
Russo I, Bubacco L, Greggio E (2012) Exosomes-associated neurodegeneration and progression of Parkinson’s disease. Am J Neurodegener Dis 1(3):217
Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F, Kou L, Yin S et al (2019) Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis 10:174
Ferrara D, Pasetto L, Bonetto V, Basso M (2018) Role of extracellular vesicles in amyotrophic lateral sclerosis. Front Neurosci 12:574
Chun, C., Smith, A. S., Bothwell, M., & Mack, D. L. (2020). The role of extracellular vesicles in the progression of ALS and their potential as biomarkers and therapeutic agents with which to combat the disease. Amyotrophic lateral sclerosis-recent advances and therapeutic challenges. IntechOpen.
Porto-Carreiro I, Février B, Paquet S, Vilette D, Raposo G (2005) Prions and exosomes: from PrPc trafficking to PrPsc propagation. Blood Cells Mol Dis 35,143–148.propagation. Blood Cells Mol Dis 35(2):143–148
Tang BL (2018) Unconventional secretion and intercellular transfer of mutant huntingtin. Cells 7:59
Agostinho P, Pliassova A, Oliveira CR, Cunha RA (2015) Localization and trafficking of amyloid-β protein precursor and secretases: Impact on Alzheimer’s disease. J Alzheimers Dis 45(2):329–347
Tam JH, Seah C, Pasternak SH (2014) The amyloid precursor protein is rapidly transported from the Golgi apparatus to the lysosome and where it is processed into beta-amyloid. Mol Brain 7(1):1–18
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189
Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW (2005) Tau, tangles, and Alzheimer’s disease. Biochim Biophys Acta 1739:216–223
Castellani RJ, Nunomura A, Lee HG, Perry G, Smith MA (2008) Phosphorylated tau: toxic, protective, or none of the above. J Alzheimers Dis 14:377–383
Polanco JC, Li C, Durisic N, Sullivan R, Gotz J (2018) Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol Commun 6:10
Cosker KE, Courchesne SL, Segal RA (2008) Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol 18(3):270–275
DeRita RM, Zerlanko B, Singh A, Lu H, Iozzo RV, Benovic JL, Languino LR (2017) c-Src, insulin-like growth factor I receptor, G-protein-coupled receptor kinases and focal adhesion kinase are enriched into prostate cancer cell exosomes. J Cell Biochem 118(1):66–73
Elsherbini A, Bieberich E (2018) Ceramide and exosomes: a novel target in cancer biology and therapy. Adv Cancer Res 140:121–154
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319(5867):1244–1247
Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E (2012) Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4). J Biol Chem 287:21384–21395
Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O et al (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18(11):1584–1593
Castillo SS, Levy M, Thaikoottathil JV, Goldkorn T (2007) Reactive nitrogen and oxygen species activate different sphingomyelinases to induce apoptosis in airway epithelial cells. Exp Cell Res 313(12):2680–2686
Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E (2014) Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 35:1792–1800
Dinkins MB, Dasgupta S, Wang G, Zhu G, He Q, Kong JN, Bieberich E (2015) The 5XFAD mouse model of Alzheimer’s disease exhibits an age-dependent increase in anti-ceramide IgG and exogenous administration of ceramide further increases anti-ceramide titers and amyloid plaque burden. J Alzheimers Dis 46(1):55–61
Falker C, Hartmann A, Guett I, Dohler F, Altmeppen H, Betzel C, Schubert R, Thurm D et al (2016) Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity. J Neurochem 137:88–100
Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI (2004) Amyloid-β peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol 164:123–131
Yang DI, Yeh CH, Chen S, Xu J, Hsu CY (2004) Neutral sphingomyelinase activation in endothelial and glial cell death induced by amyloid beta-peptide. Neurobiol Dis 17(1):99–107
Yuyama K, Sun H, Mitsutake S, Igarashi Y (2012) Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J Biol Chem 287:10977–10989
Kalra H, Drummen GPC, Mathivanan S (2016) Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci 17:170
Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19
Zhang Y, Zhao Y, Zhang L, Yu W, Wang Y, Chang W (2019) Cellular prion protein as a receptor of toxic amyloid-β42 oligomers is important for Alzheimer’s disease. Front Cell Neurosci 13:339
McAndrews KM, Kalluri R (2019) Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer 18:52
Frohlich D, Kuo WP, Fruhbeis C, Sun JJ, Zehendner CM, Luhmann HJ, Pinto S, Toedling J et al (2014) Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci 369:20130510
Fruhbeis C, Frohlich D, Kuo WP, Kramer-Albers EM (2013) Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci 7:182
Haas C, Selkoe DJ (1993) Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta peptide. Cell 75:1039–1042
Turner PR, O’Connor K, Tate WP, Abraham WC (2003) Roles of APP in regulating neuronal activity, plasticity and memory. Prog Neurobiol 70:1–32
Kaur, N., Sarkar, B., Mittal, S., Dhiman, M., Taglialatela, G., Perez-Polo, R. J., & Mantha, A. K. (2015). Oxidative stress events and neuronal dysfunction in Alzheimer’s disease: focus on APE1/Ref-1-mediated survival strategies. Free Radicals in Human Health and Disease; (pp. 175–207). Springer, New Delhi.
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81:741–766
Sharples RA, Vella LJ, Nisbet RM, Naylor R, Perez K, Barnham KJ, Hill AF (2008) Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J 22(5):1469–1478
Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, Igarashi Y (2015) A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett 589(1):84–88
Sinha MS, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, Hallbeck M (2018) Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 136(1):41–56
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA 103:11172–11177
Rajendran L, Knobloch M, Geiger KD, Dienel S, Nitsch R, Simons K, Konietzko U (2007) Increased abeta production leads to intracellular accumulation of abeta in flotillin-1-positive endosomes. Neurodegener Dis 4:164–170
Dinkins MB, Wang G, Bieberich E (2017) Sphingolipid-enriched extracellular vesicles and Alzheimer’s disease: a decade of research. J Alzheimers Dis 60:757–768
DeLeo AM, Ikezu T (2018) Extracellular vesicle biology in Alzheimer’s disease and related tauopathy. J Neuroimmune Pharmacol 13:292–308
Gendreau KL, Hall GF (2013) Tangles, toxicity, and tau secretion in AD – new approaches to a vexing problem. Front Neurol 4:160
Saman S, Kim WH, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC et al (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287:3842–3849
Guix FX (2020) The interplay between aging-associated loss of protein homeostasis and extracellular vesicles in neurodegeneration. J Neurosci Res 98:262–283
Perez M, Avila J, Hernandez F (2019) Propagation of tau via extracellular vesicles. Front Neurosci 13:698
Friesen M, Meyer-Luehmann M (2019) Aβ seeding as a tool to study cerebral amyloidosis and associated pathology. Front Mol Neurosci 12:233
Lim YJ, Lee SJ (2017) Are Exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol Commun 5:64
Langui D, Girardot N, El Hachimi KH, Allinquant B, Blanchard V, Pradier L, Duyckaerts C (2004) Subcellular topography of neuronal Abeta peptide in APPxPS1 transgenic mice. Am J Pathol 165(5):1465–1477
Quek C, Hill AF (2017) The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun 483:1178–1186
Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H et al (2002) Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161(5):1869–1879
Vingtdeux V, Hamdane M, Loyens A, Gelé P, Drobeck H, Bégard S, Galas MC, Delacourte A et al (2007) Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J Biol Chem 282(25):18197–18205
Chiarini A, Armato U, Gardenal E, Gui L, Pra ID (2017) Amyloid β-exposed human astrocytes overproduce phospho-tau and overrelease it within exosomes: effects suppressed by calcilytic NPS 2143—further implications for Alzheimer’s therapy. Front Neurosci 11:217
Yuyama K, Igarashi Y (2017) Exosomes as carriers of Alzheimer’s amyloid-ß. Front Neurosci 11:229
Guo BB, Bellingham SA, Hill AF (2015) The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem 290:3455–3467
Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H et al (2007) Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin. Appl 1:1446–1461
Trotta T, Panaro MA, Cianciulli A, Mori G, Di Benedetto A, Porro C (2018) Microglia-derived extracellular vesicles in Alzheimer’s disease: a double-edged sword. Biochem Pharmacol 148:184–192
Drago F, Lombardi M, Prada I, Gabrielli M, Joshi P, Cojoc D, Verderio C (2017) ATP modifies the proteome of extracellular vesicles released by microglia and influences their action on astrocytes. Front Pharmacol 8:910
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D (2019) Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J Neurovirol 25:702–709
Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G et al (2011) Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci 46:409–418
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25:1439–1451
Rose CF, Verkhratsky A, Parpura V (2013) Astrocyte glutamine synthetase: pivotal in health and disease. Biochem Soc Trans 41:1518–1524
Wang E, Dimova N, Cambi F (2007) PLP/DM20 ratio is regulated by hnRNPH and F and a novel G-rich enhancer in oligodendrocytes. Nucleic Acids Res 35:4164–4178
Yin X, Peterson J, Gravel M, Braun PE, Trapp BD (1997) CNP overexpression induces aberrant oligodendrocyte membranes and inhibits MBP accumulation and myelin compaction. J Neurosci Res 50:238–247
Galiano MR, Andrieux A, Deloulme JC, Bosc C, Schweitzer A, Job D, Hallak ME (2006) Myelin basic protein functions as a microtubule stabilizing protein in differentiated oligodendrocytes. J Neurosci Res 84:534–541
Peschl P, Bradl M, Hoftberger R, Berger T, Reindl M (2017) Myelin Oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol 8:529
Hopperton KE, Mohammad D, Trepanier MO, Giuliano V, Bazinet RP (2018) Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol Psychiatry 23:177–198
Roy A, Fung YK, Liu X, Pahan K (2006) Up-regulation of microglial CD11b expression by nitric oxide. J Biol Chem 281:14971–14980
Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y (2001) Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 32:1208–1215
Paolicelli RC, Bergamini G, Rajendran L (2019) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405:148–157
Delpech JC, Herron S, Botros MB, Ikezu T (2019) Neuroimmune crosstalk through extracellular vesicles in health and disease. Trends Neurosci 42(5):361–372
Guitart K, Loers G, Buck F, Bork U, Schachner M, Kleene R (2016) Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 64(6):896–910
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12(6):719–732
McQuade A, Blurton-Jones M (2019) Microglia in Alzheimer’s disease: exploring how genetics and phenotype influence risk. J Mol Biol 431(9):1805–1817
Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW et al (2008) Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 5:37
Butovsky O, Weiner HL (2018) Microglial signatures and their role in health and disease. Nat Rev Neurosci 19(10):622–635
Hamelin L, Lagarde J, Dorothée G, Leroy C, Labit M, Comley RA, Clinical IMABio3 team (2016) Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18 F-DPA-714 PET imaging. Brain 139(4):1252–1264
Gupta A, Pulliam L (2014) Exosomes as mediators of neuroinflammation. J Neuroinflammation 11:68
Brites D, Fernandes A (2015) Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci 9:476
Pascual M, Ibanez F, Guerri C (2020) Exosomes as mediators of neuron-glia communication in neuroinflammation. Neural Regen Res 15:796–801
Chen JJ, Zhao B, Zhao J, Li S (2017) Potential roles of exosomal microRNAs as diagnostic biomarkers and therapeutic application in Alzheimer’s disease. Neural Plast 2017:7027380
Li JJ, Wang B, Kodali MC, Chen C, Kim E, Patters BJ, Lan L, Kumar S et al (2018) In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflammation 15:8
Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB (2018) Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J 32(2):888–893
Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Verderio C (2012) Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J 31(5):1231–1240
De Godoy MA, Saraiva LM, de Carvalho LR, Vasconcelos-dos-Santos A, Beiral HJ, Ramos AB, Ferreira ST (2018) Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J Biol Chem 293(6):1957–1975
Wang SS, Jia J, Wang Z (2018) Mesenchymal stem cell-derived extracellular vesicles suppresses iNOS expression and ameliorates neural impairment in Alzheimer’s disease mice. J Alzheimers Dis 61(3):1005–1013
Prada I, Gabrielli M, Turola E, Iorio A, D’Arrigo G, Parolisi R, Verderio C (2018) Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol 135(4):529–550
Martínez de Arrieta C, Morte B, Coloma A, Bernal J (1999) The human RC3 gene homolog, NRGN contains a thyroid hormone-responsive element located in the first intron. Endocrinology 140(1):335–343
Pluta R, Ulamek-Kozio M, Januszewski S, J-Czuczwar S (2018) Exosomes as possible spread factor and potential biomarkers in Alzheimer’s disease: current concepts. Biomark. Med 12:1025–1033
Schoenherr CJ, Anderson DJ (1995) The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 267(5202):1360–1363
Yang TT, Liu CG, Gao SC, Zhang Y, Wang PC (2018) The serum exosome derived microRNA-135a, -193b, and -384 were potential Alzheimer’s disease Biomarkers. Biomed Environ Sci 31(2):87–96
Yin Q, Ji X, Lv R, Pei JJ, Du Y, Shen C, Hou X (2020) Targetting exosomes as a new biomarker and therapeutic approach for Alzheimer’s disease. Clin Interv Aging 15:195–205
Yuan Q, Li XD, Zhang SM, Wang HW,Wang LW (2019) Extracellular vesicles in neurodegenerative diseases: insights and new perspectives. Genes and diseases.
Wang D, Wang P, Bian X, Xu S, Zhou Q, Zhang Y, Ding M, Han M et al (2020) Elevated plasma levels of exosomal BACE1-AS combined with the volume and thickness of the right entorhinal cortex may serve as a biomarker for the detection of Alzheimer’s disease. Mol Med Rep 22:227–238
Watson LS, Hamlett ED, Stone TD, Sims-Robinson C (2019) Neuronally derived extracellular vesicles: an emerging tool for understanding Alzheimer’s disease. Mol Neurodegener 14(1):1–9
Cai ZY, Xiao M, Quazi SH, Ke ZY (2018) Exosomes: a novel therapeutic target for Alzheimer’s disease? Neural Regen Res 13:930–935
Sala M, Hollinger KR, Thomas AG, Dash RP, Tallon C, Veeravalli V, Lovell L, Kogler M et al (2020) Novel human neutral sphingomyelinase 2 inhibitors as potential therapeutics for Alzheimer’s disease. J Med Chem 63:6028–6056
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y et al (2013) Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3:1197
Iranifar E, Seresht BM, Momeni F, Fadaei E, Mehr MH, Ebrahimi Z, Rahmati M, Kharazinejad E et al (2019) Exosomes and microRNAs: new potential therapeutic candidates in Alzheimer disease therapy. J Cell Physiol 234:2296–2305
Sundelof J, Arnlov J, Ingelsson E, Sundstrom J, Basu S, Zethelius B, Larsson A, Irizarry MC et al (2008) Serum cystatin C and the risk of Alzheimer disease in elderly men. Neurology 71:1072–1079
Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J, Zhao L (2019) Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the tau protein through the AKT/GSK-3β Pathway. Nanoscale 11:7481–7496
Yang Y, Ye Y, Su X, He J, Bai W, He X (2017) MSCs-Derived Exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci 11:55
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18(9):1606–1614
Kaur, N., Sarkar, B., Gill, I., Kaur, S., Mittal, S., Dhiman, M. & Mantha, A. K. (2016). Indian herbs and their therapeutic potential against Alzheimer’s disease: what makes them special? In Neuroprotective Effects of Phytochemicals in Neurological Disorders (pp79–112). Willey Blackwell, Canada.
Kaur, S., Dhiman, M., & Mantha, A. K. (2018). Ferulic Acid: a natural antioxidant with application towards neuroprotection against Alzheimer’s disease. Functional Food and Human Health; (pp. 575–586). Springer, Singapore.
Qi Y, Guo L, Jiang Y, Shi Y, Sui S, Zhao L (2020) Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Deliv 27:745–755
An K, Klyubin I, Kim Y, Jung JH, Mably AJ, O’Dowd TS, Kim JH (2013) Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Molecular brain 6(1):1–13
Tassew NG, Charish J, Shabanzadeh AP, Luga V, Harada H, Farhani N, Monnier PP (2017) Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep 20(1):99–111
Butterfield DA, Swomley AM, Sultana R (2013) Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal 19:823–835
Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, Liu JS, Dong YR et al (2019) RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun Ageing 16:10
Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G (2004) Cells release prions in association with exosomes. Proc Natl Acad Sci USA 101:9683–9688
Yu H, Sun T, An J, Wen L, Liu F, Bu Z, Cui Y, Feng J (2020) Potential roles of exosomes in Parkinson’s disease: from pathogenesis, diagnosis, and treatment to prognosis. Front Cell Dev Biol 8:86
Zhou ZD, Chan CHS, Ma QH, Xu XH, Xiao ZC, Tan EK (2011) The roles of amyloid precursor protein (APP) in neurogenesis: implications to pathogenesis and therapy of Alzheimer disease. Cell Adh Migr 5:280–292
Saman S, Kim WH, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NCY, Hall GF (2012) Exosome-associated Tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287(6):3842–3849. https://doi.org/10.1074/jbc.M111.277061
Arscott WT, Tandle AT, Zhao S, Shabason JE, Gordon IK, Schlaff CD, Zhang G, Tofilon PJ, Camphausen KA (2013) Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Transl Oncol 6(6):638-IN6. https://doi.org/10.1593/tlo.13640
Qin, J., Xu, Q. (2014) Functions and application of exosomes. Acta Pol Pharm. 71:537-543.
Acknowledgements
H.V. and S.K. acknowledges financial support in the form of a junior/senior research fellowship (JRF/SRF) from the University Grants Commission and Council for Scientific and Industrial Research (CSIR), Govt. of India, New Delhi, India, respectively. Because of the limited focus of the article, many relevant and appropriate references could not be included, for which the authors apologize.
Author information
Authors and Affiliations
Contributions
AKM conceived the idea of the manuscript. SK and HV performed literature review and wrote the manuscript. MD made the outline of the draft. SK and HV made figures and table. GT, GLG, and FJ critically reviewed the manuscript and helped in revising it. All authors have read the manuscript and agreed to the final draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sharanjot Kaur and Harkomal Verma: These authors contributed equally to the work
Rights and permissions
About this article
Cite this article
Kaur, S., Verma, H., Dhiman, M. et al. Brain Exosomes: Friend or Foe in Alzheimer’s Disease?. Mol Neurobiol 58, 6610–6624 (2021). https://doi.org/10.1007/s12035-021-02547-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02547-y