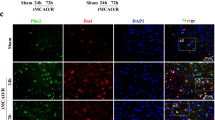
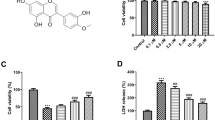
Abstract
Ischemic stroke is an inflammation-related disease, during which process activation of NLRP3 inflammasome and subsequent pyroptosis play crucial roles. Platelet-activating factor (PAF) is a potent phospholipid regulator of inflammation which exerts its effect via binding specific PAF receptor (PAFR). However, whether PAFR contributes to pyroptosis during ischemia/reperfusion (I/R) injury remains to be elucidated. To explore the underlying effect of PAFR on ischemic stroke from the perspective of pyroptosis, mice were subjected to middle cerebral artery occlusion/reperfusion (MCAO/R) injury and primary cultures of mice cerebral cortical neurons were exposed to oxygen–glucose deprivation/reoxygenation (OGD/R) injury to mimic I/R in vivo and in vitro, after which indexes associated with pyroptosis were analyzed. Intriguingly, our results indicated that inhibition of PAFR with its inhibitor XQ-1H or PAFR siRNA exerted a neuroprotective effect against I/R injury both in vivo and in vitro. Furthermore, inflammasome activation and pyroptosis after ischemic challenge were attenuated by XQ-1H or PAFR siRNA. Besides, the protection of XQ-1H was abolished by PAF stimulaiton to some extent. Moreover, XQ-1H or PAFR siRNA alleviated the neuronal pyroptosis induced by LPS and nigericin (an NLRP3 activator) in cortical neurons. Taken together, this study firstly demonstrates that PAFR is involved in neuronal pyroptosis after I/R injury, and XQ-1H, a specific PAFR inhibitor, has a promising prospect in attenuating I/R injury from the perspective of anti-pyroptosis.
Graphical abstract












Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
Imai T, Matsubara H, Nakamura S, Hara H, Shimazawa M (2020) The mitochondria-targeted peptide, bendavia, attenuated ischemia/reperfusion-induced stroke damage. Neuroscience 443:110–119. https://doi.org/10.1016/j.neuroscience.2020.07.044
Yang B, Sun Y, Lv C, Zhang W, Chen Y (2020) Procyanidins exhibits neuroprotective activities against cerebral ischemia reperfusion injury by inhibiting TLR4-NLRP3 inflammasome signal pathway. Psychopharmacology 237(11):3283–3293. https://doi.org/10.1007/s00213-020-05610-z
Huang Y, Liu Z, Tan F, Hu Z, Lu M (2020) Effects of the insulted neuronal cells-derived extracellular vesicles on the survival of umbilical cord-derived mesenchymal stem cells following cerebral ischemia/reperfusion injury. Oxid Med Cell Longev 2020:9768713. https://doi.org/10.1155/2020/9768713
Guo Z, Yu S, Chen X, Ye R, Zhu W, Liu X (2016) NLRP3 is involved in ischemia/reperfusion injury. CNS Neurol Disord Drug Targets 15(6):699–712. https://doi.org/10.2174/1871527315666160321111829
Han C, Yang Y, Guan Q, Zhang X, Shen H, Sheng Y, Wang J, Zhou X, Li W, Guo L, Jiao Q (2020) New mechanism of nerve injury in Alzheimer’s disease: β-amyloid-induced neuronal pyroptosis. J Cell Mol Med 24(14):8078–8090. https://doi.org/10.1111/jcmm.15439
Sita G, Graziosi A, Hrelia P, Morroni F (2021) NLRP3 and infections: β-amyloid in inflammasome beyond neurodegeneration. Int J Mol Sci 22(13):6984. https://doi.org/10.3390/ijms22136984
Hanslik KL, Ulland TK (2020) The role of microglia and the Nlrp3 inflammasome in Alzheimer’s disease. Front Neurol 11:570711. https://doi.org/10.3389/fneur.2020.570711
Weber ANR, Bittner ZA, Shankar S, Liu X, Chang TH, Jin T, Tapia-Abellán A (2020) Recent insights into the regulatory networks of NLRP3 inflammasome activation. J Cell Sci 133(23): jcs248344. https://doi.org/10.1242/jcs.248344
Feng YS, Tan ZX, Wang MM, Xing Y, Dong F, Zhang F (2020) Inhibition of NLRP3 Inflammasome: a prospective target for the treatment of ischemic stroke. Front Cell Neurosci 14(155). https://doi.org/10.3389/fncel.2020.00155
Feng YS, Tan ZX, Wu LY, Dong F, Zhang F (2021) The involvement of NLRP3 inflammasome in the treatment of neurodegenerative diseases. Biomed Pharmacother 138:111428. https://doi.org/10.1016/j.biopha.2021.111428
Poh L, Kang SW, Baik SH, Ng GYQ, She DT, Balaganapathy P, Dheen ST, Magnus T, Gelderblom M, Sobey CG, Koo EH, Fann DY, Arumugam TV (2019) Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun 75:34–47. https://doi.org/10.1016/j.bbi.2018.09.001
Tian Z, Chu T, Shields LBE, Zhu Q, Zhang YP, Kong M, Barnes GN, Wang Y, Shields CB, Cai J (2020) Platelet-activating factor deteriorates lysophosphatidylcholine-induced demyelination via its receptor-dependent and -independent effects. Mol Neurobiol 57(10):4069–4081. https://doi.org/10.1007/s12035-020-02003-3
Li X, Huang L, Liu G, Fan W, Li B, Liu R, Wang Z, Fan Q, Xiao W, Li Y, Fang W (2020) Ginkgo diterpene lactones inhibit cerebral ischemia/reperfusion induced inflammatory response in astrocytes via TLR4/NF-κB pathway in rats. J Ethnopharmacol 249:112365. https://doi.org/10.1016/j.jep.2019.112365
Liu Y, Shields LBE, Gao Z, Wang Y, Zhang YP, Chu T, Zhu Q, Shields CB, Cai J (2017) Current understanding of platelet-activating factor signaling in central nervous system diseases. Mol Neurobiol 54(7):5563–5572. https://doi.org/10.1007/s12035-016-0062-5
Toscano EC, Silva BC, Victoria EC, Cardoso AC, Miranda AS, Sugimoto MA, Sousa LP, Carvalho BA, Kangussu LM, Silva DG, Rodrigues FG, Barcelos Lda S, Vasconcelos AC, Amaral FA, Teixeira MM, Teixeira AL, Rachid MA (2016) Platelet-activating factor receptor (PAFR) plays a crucial role in experimental global cerebral ischemia and reperfusion. Brain Res Bull 124:55–61. https://doi.org/10.1016/j.brainresbull.2016.03.022
Wang EW, Han YY, Jia XS (2018) PAFR-deficiency alleviates myocardial ischemia/reperfusion injury in mice via suppressing inflammation, oxidative stress and apoptosis. Biochem Biophys Res Commun 495(4):2475–2481. https://doi.org/10.1016/j.bbrc.2017.12.132
Liu G, Mateer SW, Hsu A, Goggins BJ, Tay H, Mathe A, Fan K, Neal R, Bruce J, Burns G, Minahan K, Maltby S, Fricker M, Foster PS, Wark PAB, Hansbro PM, Keely S (2019) Platelet activating factor receptor regulates colitis-induced pulmonary inflammation through the NLRP3 inflammasome. Mucosal Immunol 12(4):862–873. https://doi.org/10.1038/s41385-019-0163-3
Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH (2010) Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke 41(8):1783–1790. https://doi.org/10.1161/strokeaha.110.586537
Longa EZ, Weinstein PR, Carlson S, Cummins CR (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91. https://doi.org/10.1161/01.str.20.1.84
Fei YX, Zhu JP, Zhao B, Yin QY, Fang WR, Li YM (2020) XQ-1H regulates Wnt/GSK3β/β-catenin pathway and ameliorates the integrity of blood brain barrier in mice with acute ischemic stroke. Brain Res Bull 164:269–288. https://doi.org/10.1016/j.brainresbull.2020.08.032
Deng M, Guo H, Tam JW, Johnson BM, Brickey WJ, New JS, Lenox A, Shi H, Golenbock DT, Koller BH, McKinnon KP, Beutler B, Ting JP (2019) Platelet-activating factor (PAF) mediates NLRP3-NEK7 inflammasome induction independently of PAFR. J Exp Med 216(12):2838–2853. https://doi.org/10.1084/jem.20190111
Vital SA, Gavins FN (2016) Surgical approach for middle cerebral artery occlusion and reperfusion induced stroke in mice. J Vis Exp 20(116):54302. https://doi.org/10.3791/54302
Yang L, Chen X, Wang S, Fei Y, Wang D, Li Y, He G, Wu Q, Chu S, Fang W (2015) N2 extenuates experimental ischemic stroke through platelet aggregation inhibition. Thromb Res 136(6):1310–1317. https://doi.org/10.1016/j.thromres.2015.10.039
Zhao B, Zhu J, Fei Y, Yin Q, Shen W, Liang B, Zhu X, Li Y (2020) JLX001 attenuates blood-brain barrier dysfunction in MCAO/R rats via activating the Wnt/β-catenin signaling pathway. Life Sci 260:118221. https://doi.org/10.1016/j.lfs.2020.118221
Del Bel EA, Souza AS, Guimarães FS, da Silva CA, Nucci-da-Silva LP (2002) Motor effects of acute and chronic inhibition of nitric oxide synthesis in mice. Psychopharmacology (Berl) 161(1):32–37. https://doi.org/10.1007/s00213-002-1009-2
Deng Y, Fang W, Li Y, Cen J, Fang F, Lv P, Gong S, Mao L (2009) Blood-brain barrier breakdown by PAF and protection by XQ-1H due to antagonism of PAF effects. Eur J Pharmacol 616(1):43–47. https://doi.org/10.1016/j.ejphar.2009.06.017
Huang J, Lu W, Doycheva DM, Gamdzyk M, Hu X, Liu R, Zhang JH, Tang J (2020) IRE1α inhibition attenuates neuronal pyroptosis via miR-125/NLRP1 pathway in a neonatal hypoxic-ischemic encephalopathy rat model. J Neuroinflammation 17(1):152. https://doi.org/10.1186/s12974-020-01796-3
Li J, Hao JH, Yao D, Li R, Li XF, Yu ZY, Luo X, Liu XH, Wang MH, Wang W (2020) Caspase-1 inhibition prevents neuronal death by targeting the canonical inflammasome pathway of pyroptosis in a murine model of cerebral ischemia. CNS Neurosci Ther 26(9):925–939. https://doi.org/10.1111/cns.13384
Liang YB, Song PP, Zhu YH, Xu JM, Zhu PZ, Liu RR, Zhang YS (2020) TREM-1-targeting LP17 attenuates cerebral ischemia-induced neuronal injury by inhibiting oxidative stress and pyroptosis. Biochem Biophys Res Commun 529(3):554–561. https://doi.org/10.1016/j.bbrc.2020.05.056
Yuan YY, Xie KX, Wang SL, Yuan LW (2018) Inflammatory caspase-related pyroptosis: mechanism, regulation and therapeutic potential for inflammatory bowel disease. Gastroenterol Rep 6(3):167–176. https://doi.org/10.1093/gastro/goy011
Wang J, Deng B, Liu Q, Huang Y, Chen W, Li J, Zhou Z, Zhang L, Liang B, He J, Chen Z, Yan C, Yang Z, Xian S, Wang L (2020) Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis 11(7):574. https://doi.org/10.1038/s41419-020-02777-3
Ding HG, Li Y, Li XS, Liu XQ, Wang KR, Wen MY, Jiang WQ, Zeng HK Hypercapnia promotes microglial pyroptosis via inhibiting mitophagy in hypoxemic adult rats. CNS Neurosci Ther 26(11):1134–1146. https://doi.org/10.1111/cns.13435
Peng D, Li J, Deng Y, Zhu X, Zhao L, Zhang Y, Li Z, Ou S, Li S, Jiang Y (2020) Sodium para-aminosalicylic acid inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis by inhibiting NF-κB pathway activation and oxidative stress. J Neuroinflammation 17(1):343. https://doi.org/10.1186/s12974-020-02018-6
Tang YS, Zhao YH, Zhong Y, Li XZ, Pu JX, Luo YC, Zhou QL (2019) Neferine inhibits LPS-ATP-induced endothelial cell pyroptosis via regulation of ROS/NLRP3/Caspase-1 signaling pathway. Inflamm Res 68(9):727–738. https://doi.org/10.1007/s00011-019-01256-6
Wang J, Yao J, Liu Y, Huang L (2021) Targeting the gasdermin D as a strategy for ischemic stroke therapy. Biochem Pharmacol 188:114585. https://doi.org/10.1016/j.bcp.2021.114585
Gou X, Xu D, Li F, Hou K, Fang W, Li Y (2021) Pyroptosis in stroke-new insights into disease mechanisms and therapeutic strategies. J Physiol Biochem. https://doi.org/10.1007/s13105-021-00817-w
Ma DC, Zhang NN, Zhang YN, Chen HS (2020) Kv1.3 channel blockade alleviates cerebral ischemia/reperfusion injury by reshaping M1/M2 phenotypes and compromising the activation of NLRP3 inflammasome in microglia. Exp Neurol 332: 113399. https://doi.org/10.1016/j.expneurol.2020.113399
Wang QS, Ding HG, Chen SL, Liu XQ, Deng YY, Jiang WQ, Li Y, Huang LQ, Han YL, Wen MY, Wang MQ, Zeng HK (2020) Hypertonic saline mediates the NLRP3/IL-1β signaling axis in microglia to alleviate ischemic blood-brain barrier permeability by downregulating astrocyte-derived VEGF in rats. CNS Neurosci Ther 26(10):1045–1057. https://doi.org/10.1111/cns.13427
Wang K, Ru J, Zhang H, Chen J, Lin X, Lin Z, Wen M, Huang L, Ni H, Zhuge Q, Yang S (2020) Melatonin enhances the therapeutic effect of plasma exosomes against cerebral ischemia-induced pyroptosis through the TLR4/NF-κB pathway. Front Neurosci 14 (848). https://doi.org/10.3389/fnins.2020.00848
Zhou Y, Gu Y, Liu J (2019) BRD4 suppression alleviates cerebral ischemia-induced brain injury by blocking glial activation via the inhibition of inflammatory response and pyroptosis. Biochem Biophys Res Commun 519(3):481–488. https://doi.org/10.1016/j.bbrc.2019.07.097
Hug S, Bernhard S, Stratmann AEP, Erber M, Wohlgemuth L, Knapp CL, Bauer JM, Vidoni L, Fauler M, Föhr KJ, Radermacher P, Hoffmann A, Huber-Lang M, Messerer DAC (2021) Activation of neutrophil granulocytes by platelet-activating factor is impaired during experimental sepsis. Front Immunol 12(580). https://doi.org/10.3389/fimmu.2021.642867
Lordan R, Tsoupras A, Zabetakis I, Demopoulos CA (2019) Forty years since the structural elucidation of platelet-activating factor (PAF): historical, current, and future research perspectives. Molecules 24(23):4414. https://doi.org/10.3390/molecules24234414
Souza DG, Pinho V, Soares AC, Shimizu T, Ishii S, Teixeira MM (2003) Role of PAF receptors during intestinal ischemia and reperfusion injury. A comparative study between PAF receptor-deficient mice and PAF receptor antagonist treatment. Br J Pharmacol 139(4):733–740. https://doi.org/10.1038/sj.bjp.0705296
Yin XJ, Chen ZY, Zhu XN, Hu JJ (2017) Loss of PAFR prevents neuroinflammation and brain dysfunction after traumatic brain injury. Sci Rep 7(1):40614. https://doi.org/10.1038/srep40614
McKenzie BA, Fernandes JP, Doan MAL, Schmitt LM, Branton WG, Power C (2020) Activation of the executioner caspases-3 and -7 promotes microglial pyroptosis in models of multiple sclerosis. J Neuroinflammation 17(1):253. https://doi.org/10.1186/s12974-020-01902-5
Acknowledgements
The authors acknowledge the technical support from Cellular and Molecular Biology Center of China Pharmaceutical University.
Funding
This work was supported by Natural Science Foundation of China (Program No. 82073845) and the National Major Scientific and Technological Special Project for “Significant New Drugs Development” during the Thirteenth Five-year Plan Period (No. 2019ZX09301-134 and No. 2016ZX09101031, respectively).
Author information
Authors and Affiliations
Contributions
Yunman Li and Weirong Fang conceived the whole work design; Bo Zhao and Yuxiang Fei performed the experiments and wrote the manuscript; Jianping Zhu and Qiyang Yin helped carry out the experiments. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were approved by the Animal Ethics Committee of China Pharmaceutical University [license number: SYXK (Su) 2016–0011].
Consent to Participate
Not applicable to this study.
Consent for Publication
Not applicable to this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, B., Fei, Y., Zhu, J. et al. PAF Receptor Inhibition Attenuates Neuronal Pyroptosis in Cerebral Ischemia/Reperfusion Injury. Mol Neurobiol 58, 6520–6539 (2021). https://doi.org/10.1007/s12035-021-02537-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02537-0