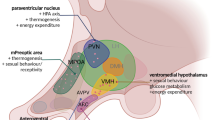
Abstract
The stress response is multifactorial and enrolls circuitries to build a coordinated reaction, leading to behavioral, endocrine, and autonomic changes. These changes are mainly related to the hypothalamus–pituitary–adrenal (HPA) axis activation and the organism’s integrity. However, when self-regulation is ineffective, stress becomes harmful and predisposes the organism to pathologies. The chronic unpredictable stress (CUS) is a widely used experimental model since it induces physiological and behavioral changes and better mimics the stressors variability encountered in daily life. Corticotropin-releasing factor (CRF) and glucocorticoids (GCs) are deeply implicated in the CUS-induced physiological and behavioral changes. Nonetheless, the CUS modulation of CRF receptors and GR and the norepinephrine role in extra-hypothalamic brain areas were not well explored. Here, we show that 14 days of CUS induced a long-lasting HPA axis hyperactivity evidenced by plasmatic corticosterone increase and adrenal gland hypertrophy, which was dependent on both GCs and NE release induced by each stress session. CUS also increased CRF2 mRNA expression and GR protein levels in fundamental brain structures related to HPA regulation and behavior, such as the lateral septal nucleus intermedia part (LSI), ventromedial hypothalamic nucleus (VMH), and central nucleus of the amygdala (CeA). We also showed that NE participates in the CUS-induced increase in CRF2 and GR levels in the LSI, reinforcing the locus coeruleus (LC) involvement in the HPA axis modulation. Despite the CUS-induced molecular changes in essential areas related to anxiety-like behavior, this phenotype was not observed in CUS animals 24 h after the last stress session.







Similar content being viewed by others
Data Availability (Data Transparency)
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code Availability (Software Application or Custom Code)
Not applicable.
References
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10(6):397–409
de Kloet ER (2008) About stress hormones and resilience to psychopathology. J Neuroendocrinol 20(6):885–892
Goldstein DS, McEwen B (2002) Allostasis, homeostats, and the nature of stress. Stress 5(1):55–58
Bejean S, Sultan-Taieb H (2005) Modeling the economic burden of diseases imputable to stress at work. Eur J Health Econ 6(1):16–23
de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–475
Van de Kar LD, Blair ML (1999) Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol 20(1):1–48
Akil HA, Morano MI (1995) Stress. In: Bloom FE, Kupfer DJ (eds) psychopharmacology: the fourth generation of progress. Raven Press, New York, pp 773–785
Romier C et al (1993) Solution of structure of human corticotropin releasing factor by HNMR and distance geometry with restrained molecular dynamics. Prot Eng 6:149–156
Trainer PJ et al (1995) A comparison of the effects of human and ovine corticotropin-releasing hormone on the pituitary-adrenal axis. J Clin Endocrinol Metab 80(2):412–417
Hillhouse EW, Grammatopoulos DK (2006) The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 27(3):260–286
Dunn AJ, Swiergiel AH, Palamarchouk V (2004) Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci 1018:25–34
Gallagher JP et al (2008) Synaptic physiology of central CRH system. Eur J Pharmacol 583(2–3):215–225
Henckens MJ, Deussing JM, Chen A (2016) Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat Rev Neurosci 17(10):636–651
Shekhar A et al (2005) Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 8(4):209–219
Bale TL et al (2000) Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24(4):410–414
Coste SC et al (2000) Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24(4):403–409
Kozicz T et al (2004) Urocortin expression in the Edinger-Westphal nucleus is down-regulated in transgenic mice over-expressing neuronal corticotropin-releasing factor. Neuroscience 123(3):589–594
Reul JM, Holsboer F (2002) Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol 2(1):23–33
Bale TL, Vale WW (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557
Ingallinesi M et al (2012) CRF2 receptor-deficiency eliminates opiate withdrawal distress without impairing stress coping. Mol Psychiatry 17(12):1283–1294
Carrasco GA, Van de Kar LD (2003) Neuroendocrine pharmacology of stress. Eur J Pharmacol 463(1–3):235–272
Keller-Wood M (2015) Hypothalamic-pituitary-adrenal axis—feedback control. Compr Physiol 5(3):1161–1182
McEwen BS (1998) Protective and damaging effects of stress mediators. N Engl J Med 338:171–179
McEwen BS, Sapolsky RM (1995) Stress and cognitive function. Curr Opin Neurobiol 5(2):205–216
De Kloet ER, Derijk R (2004) Signaling pathways in brain involved in predisposition and pathogenesis of stress-related disease: genetic and kinetic factors affecting the MR/GR balance. Ann N Y Acad Sci 1032:14–34
Willner P (2017) The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress 6:78–93
Joels M et al (2004) Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress 7(4):221–231
Willner P (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52(2):90–110
Munhoz CD et al (2006) Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci 26(14):3813–3820
Tata DA, Yamamoto BK (2008) Chronic stress enhances methamphetamine-induced extracellular glutamate and excitotoxicity in the rat striatum. Synapse 62(5):325–336
Garcia-Bueno B et al (2008) Stress mediators regulate brain prostaglandin synthesis and peroxisome proliferator-activated receptor-gamma activation after stress in rats. Endocrinology 149(4):1969–1978
Simmons DM, Arriza JL, Swanson LW (1989) A Complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labeled single-stranded RNA probes. J Histotechnol 12(3):169–181
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29(4):577–580
Conover WJ (1999) Pratical nonparametric statistics, 3rd edn. Wiley, Hoboken
Bakshi VP et al (2007) Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J Neurosci 27(39):10568–10577
Jeanneteau FD et al (2012) BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci U S A 109(4):1305–1310
Sapolsky R (2003) Taming stress. Sci Am 289(3):86–95
Kling MA, Coleman VH, Schulkin J (2009) Glucocorticoid inhibition in the treatment of depression: can we think outside the endocrine hypothalamus? Depress Anxiety 26(7):641–649
Herman JP et al (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24(3):151–180
Koob GF (1999) Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 46(9):1167–1180
Korosi A, Baram TZ (2008) The central corticotropin releasing factor system during development and adulthood. Eur J Pharmacol 583(2–3):204–214
Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20(2):78–84
Flak JN et al (2014) Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci 39(11):1903–1911
Wang B et al (2017) Effects of alpha2A adrenoceptors on norepinephrine secretion from the locus coeruleus during chronic stress-induced depression. Front Neurosci 11:243
Agon P et al (1991) Permeability of the blood-brain barrier for atenolol studied by positron emission tomography. J Pharm Pharmacol 43(8):597–600
Madrigal JL et al (2002) Stress-induced increase in extracellular sucrose space in rats is mediated by nitric oxide. Brain Res 938(1–2):87–91
Radley JJ, Johnson SB, Sawchenko PE (2017) Stress: neuroendocrinology and neurobiology. In: Fink G (ed) Handbook of Stress Series. Academic Press, New York, pp 17–27
Morilak DA et al (2005) Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 29(8):1214–1224
Herman JP et al (2005) Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29(8):1201–1213
Singewald GM et al (2011) The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology 36(4):793–804
Gold PW (2015) The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 20(1):32–47
van Bodegom M, Homberg JR, Henckens M (2017) Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 11:87
Henry B, Vale W, Markou A (2006) The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci 26(36):9142–9152
Heinrichs M et al (2003) Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 54(12):1389–1398
Southwick SM, Vythilingam M, Charney DS (2005) The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol 1:255–291
Sterling P, Eyer J (1988) Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J (eds) Handbook of life stress, cognition and health. Wiley, New York, pp 629–649
Acknowledgements
We gratefully thank Guiomar Wiesel and Larissa de Sá Lima for technical assistance.
Funding
This article was supported by research grants to J.B. from Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo Research Foundation — FAPESP) grants #2010/52068–0, #2016/02224–1. J.B. was also supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Agency for the Advancement of Higher Education) and Comité Français d’Evaluation de la Coopération Universitaire avec le Brésil (French Committee for the Evaluation of Academic and Scientific Cooperation with Brazil) grant CAPES-COFECUB 848/15. J.B. is an Investigator with the Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development— CNPq) with grant # #426378/2016–4. This work was supported by research grants to C.D.M. from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP: 2008/55178–0, 2012/24727–4, and 2016/03572–3) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 479153/2009–4 and 422523/2016–0). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. M.B.M. was supported by FAPESP (2006/52566–4) and CAPES. L.S.N. was supported by FAPESP (2010/13843–8 and 2012/24002–0). N.B.S. was supported by CNPq (160570/2012–3); C.D.M., C.S., and R.C. are research fellows from CNPq.
Author information
Authors and Affiliations
Contributions
M.B.M. designed research, performed research, analyzed data, wrote the paper, revised the paper final version; J.M. designed research, performed research, revised the paper final version; L.S.N. performed research, analyzed data, revised the paper final version; N.B.S. performed research, analyzed data, revised the paper final version; L.S. analyzed data, contributed unpublished reagents/analytic tools, revised the paper final version; R.C: contributed unpublished reagents/analytic tools, revised the paper final version; C.S. contributed unpublished reagents/analytic tools, analyzed data, revised the paper final version; J.B. contributed unpublished reagents/analytic tools, analyzed data, revised the paper final version; C.D.M.: designed research, analyzed data, contributed unpublished reagents/analytic tools, wrote the paper and revised the paper final version.
Corresponding author
Ethics declarations
Ethics Approval
All animals were used following the standards of the Ethics Committee for Animal Use of the Institute of Biomedical Sciences/University of São Paulo (CEUA- ICB 102/06 and 75/05) and the guidelines of the Brazilian National Council for the Control of Animal Experimentation (CONCEA).
Consent to Participate
Not applicable.
Consent for Publication
All authors have contributed significantly and agreed with the entire content of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malta, M.B., Martins, J., Novaes, L.S. et al. Norepinephrine and Glucocorticoids Modulate Chronic Unpredictable Stress-Induced Increase in the Type 2 CRF and Glucocorticoid Receptors in Brain Structures Related to the HPA Axis Activation. Mol Neurobiol 58, 4871–4885 (2021). https://doi.org/10.1007/s12035-021-02470-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02470-2