Abstract
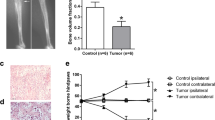
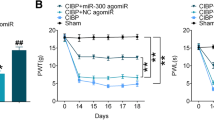
Bone cancer pain (BCP) was associated with microRNA dysregulation. In this study, we intended to clarify the potential role of miR-135-5p in a BCP mouse model, which was established by tumor cell implantation (TCI) in the medullary cavity of the mouse femur. The BCP-related behaviors were tested, including the paw withdrawal mechanical threshold (PWMT) and number of spontaneous flinches (NSF). The miRNA expression profiles in astrocytes of the sham and tumor groups were compared, and miRNA microarray and quantitative real-time PCR (qRT-PCR) assays confirmed that the amount of expression of miR-135-5p was significantly decreased in astrocytes of the tumor group. Gain- and loss-of-function studies showed that miR-135-5p could inhibit astrocyte activation and inflammation cytokine (TNF-α and IL-1β) expression. The relation between miR-135-5p and JAK2 was detected by bioinformatic analysis and dual luciferase reporter gene assay. By conducting in vitro experiments, it was shown that the miR-135-5P mimics lowered the level of JAK2/STAT3 proteins and inflammatory factors in astrocytes. Moreover, in vivo analysis on BCP mice model indicated that the miR-135-5p agonist could sufficiently increase PWMT and decrease NSF. Meanwhile, reduced activation of astrocytes in the spinal cord, as well as decreased expression of JAK2/STAT3 and inflammatory mediators, were found after miR-135-5p agonist treatment. Collectively, the results showed that miR-135-5p could potentially reduce BCP in mice through inhibiting astrocyte-mediated neuroinflammation and blocking of the JAK2/STAT3 signaling pathway, indicating that the upregulation of miR-135-5P could be a therapeutic focus in BCP treatment.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2(8):584–593. https://doi.org/10.1038/nrc867
Delaney A, Fleetwood-Walker SM, Colvin LA, Fallon M (2008) Translational medicine: cancer pain mechanisms and management. Br J Anaesth 101(1):87–94. https://doi.org/10.1093/bja/aen100
Liu S, Liu YP, Lv Y, Yao JL, Yue DM, Zhang MY, Qi DY, Liu GJ (2018) IL-18 contributes to bone cancer pain by regulating glia cells and neuron interaction. The journal of pain : official journal of the American Pain Society 19(2):186–195. https://doi.org/10.1016/j.jpain.2017.10.003
Zhao D, Han DF, Wang SS, Lv B, Wang X, Ma C (2019) Roles of tumor necrosis factor-alpha and interleukin-6 in regulating bone cancer pain via TRPA1 signal pathway and beneficial effects of inhibition of neuro-inflammation and TRPA1. Mol Pain 15:1744806919857981. https://doi.org/10.1177/1744806919857981
Elramah S, Lopez-Gonzalez MJ, Bastide M, Dixmerias F, Roca-Lapirot O, Wielanek-Bachelet AC, Vital A, Leste-Lasserre T, Brochard A, Landry M, Favereaux A (2017) Spinal miRNA-124 regulates synaptopodin and nociception in an animal model of bone cancer pain. Sci Rep 7(1):10949. https://doi.org/10.1038/s41598-017-10224-1
Hou B, Cui X, Liu Y, Zhang W, Liu M, Sun YE, Ma Z, Gu X (2016) Positive feedback regulation between microRNA-132 and CREB in spinal cord contributes to bone cancer pain in mice. Eur J Pain 20(8):1299–1308. https://doi.org/10.1002/ejp.854
Gandla J, Lomada SK, Lu J, Kuner R, Bali KK (2017) miR-34c-5p functions as pronociceptive microRNA in cancer pain by targeting Cav2. 3 containing calcium channels. Pain 158(9):1765–1779. https://doi.org/10.1097/j.pain.0000000000000971
Yu SN, Liu GF, Li LY, Zhao GQ, Liu L, Li XF (2019) Analgesic effects of microRNA-129-5p against bone cancer pain through the EphB1/EphrinB2 signaling pathway in mice. J Cell Biochem 120(3):2876–2885. https://doi.org/10.1002/jcb.26605
Meza-Sosa KF, Pedraza-Alva G, Perez-Martinez L (2014) microRNAs: key triggers of neuronal cell fate. Front Cell Neurosci 8:175. https://doi.org/10.3389/fncel.2014.00175
van Battum EY, Verhagen MG, Vangoor VR, Fujita Y, Derijck A, O’Duibhir E, Giuliani G, de Gunst T, Adolfs Y, Lelieveld D, Egan D, Schaapveld RQJ, Yamashita T, Pasterkamp RJ (2018) An image-based miRNA screen identifies miRNA-135s as regulators of CNS axon growth and regeneration by targeting Kruppel-like Factor 4. The Journal of neuroscience : the official journal of the Society for Neuroscience 38(3):613–630. https://doi.org/10.1523/JNEUROSCI.0662-17.2017
Wang N, Yang Y, Pang M, Du C, Chen Y, Li S, Tian Z, Feng F, Wang Y, Chen Z, Liu B, Rong L (2020) MicroRNA-135a-5p promotes the functional recovery of spinal cord injury by targeting SP1 and ROCK. Molecular therapy Nucleic acids 22:1063–1077. https://doi.org/10.1016/j.omtn.2020.08.035
Chiang CY, Sessle BJ, Dostrovsky JO (2012) Role of astrocytes in pain. Neurochem Res 37(11):2419–2431. https://doi.org/10.1007/s11064-012-0801-6
Lu C, Liu Y, Sun B, Sun Y, Hou B, Zhang Y, Ma Z, Gu X (2015) Intrathecal injection of JWH-015 attenuates bone cancer pain via time-dependent modification of pro-inflammatory cytokines expression and astrocytes activity in spinal cord. Inflammation 38(5):1880–1890. https://doi.org/10.1007/s10753-015-0168-3
Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K (2011) JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain : a journal of neurology 134(Pt 4):1127–1139. https://doi.org/10.1093/brain/awr025
Zhao H, Alam A, Chen Q, Eusman MA, Pal A, Eguchi S, Wu L, Ma D (2017) The role of microglia in the pathobiology of neuropathic pain development: what do we know? British journal of anaesthesia 118(4):504–516. https://doi.org/10.1093/bja/aex006
Li T, Chen X, Zhang C, Zhang Y, Yao W (2019) An update on reactive astrocytes in chronic pain. J Neuroinflammation 16(1):140. https://doi.org/10.1186/s12974-019-1524-2
Ji RR, Donnelly CR, Nedergaard M (2019) Astrocytes in chronic pain and itch. Nat Rev Neurosci 20(11):667–685. https://doi.org/10.1038/s41583-019-0218-1
Gao YJ, Ji RR (2010) Targeting astrocyte signaling for chronic pain. Neurotherapeutics 7(4):482–493. https://doi.org/10.1016/j.nurt.2010.05.016
Sommer C, Leinders M, Uceyler N (2018) Inflammation in the pathophysiology of neuropathic pain. Pain 159(3):595–602. https://doi.org/10.1097/j.pain.0000000000001122
Ellis A, Bennett DL (2013) Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 111(1):26–37. https://doi.org/10.1093/bja/aet128
Chen SP, Zhou YQ, Liu DQ, Zhang W, Manyande A, Guan XH, Tian YK, Ye DW, Omar DM (2017) PI3K/Akt pathway: a potential therapeutic target for chronic pain. Curr Pharm Des 23(12):1860–1868. https://doi.org/10.2174/1381612823666170210150147
Zhang J, Wang L, Wang H, Su Z, Pang X (2019) Neuroinflammation and central PI3K/Akt/mTOR signal pathway contribute to bone cancer pain. Mol Pain 15:1744806919830240. https://doi.org/10.1177/1744806919830240
Grenald SA, Doyle TM, Zhang H, Slosky LM, Chen Z, Largent-Milnes TM, Spiegel S, Vanderah TW, Salvemini D (2017) Targeting the S1P/S1PR1 axis mitigates cancer-induced bone pain and neuroinflammation. Pain 158(9):1733–1742. https://doi.org/10.1097/j.pain.0000000000000965
Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M (2008) JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem 107(1):50–60. https://doi.org/10.1111/j.1471-4159.2008.05566.x
Liu S, Mi WL, Li Q, Zhang MT, Han P, Hu S, Mao-Ying QL, Wang YQ (2015) Spinal IL-33/ST2 Signaling contributes to neuropathic pain via neuronal CaMKII-CREB and astroglial JAK2-STAT3 cascades in mice. Anesthesiology 123(5):1154–1169. https://doi.org/10.1097/ALN.0000000000000850
Liu S, Li Q, Zhang MT, Mao-Ying QL, Hu LY, Wu GC, Mi WL, Wang YQ (2016) Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1beta via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci Rep 6:28956. https://doi.org/10.1038/srep28956
Wang ZF, Li Q, Liu SB, Mi WL, Hu S, Zhao J, Tian Y, Mao-Ying QL, Jiang JW, Ma HJ, Wang YQ, Wu GC (2014) Aspirin-triggered lipoxin A4 attenuates mechanical allodynia in association with inhibiting spinal JAK2/STAT3 signaling in neuropathic pain in rats. Neuroscience 273:65–78. https://doi.org/10.1016/j.neuroscience.2014.04.052
Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW (1999) Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 19(24):10886–10897
Wu XP, Yang YP, She RX, Xing ZM, Chen HW, Zhang YW (2019) microRNA-329 reduces bone cancer pain through the LPAR1-dependent LPAR1/ERK signal transduction pathway in mice. Therapeutic advances in medical oncology 11:1758835919875319. https://doi.org/10.1177/1758835919875319
Zhu C, Wang K, Chen Z, Han Y, Chen H, Li Q, Liu Z, Qian L, Tang J, Shen H (2020) Antinociceptive effect of intrathecal injection of miR-9-5p modified mouse bone marrow mesenchymal stem cells on a mouse model of bone cancer pain. J Neuroinflammation 17(1):85. https://doi.org/10.1186/s12974-020-01765-w
Liu Y, Liao S, Quan H, Lin Y, Li J, Yang Q (2016) Involvement of microRNA-135a-5p in the protective effects of hydrogen sulfide against Parkinson’s disease. Cell Physiol Biochem 40(1 2):18–26. https://doi.org/10.1159/000452521
He Y, Wu L, Dai Y, Li J, Liu S (2019) MicroRNA-135 inhibits gastric cancer metastasis by targeting SMAD2. Eur Rev Med Pharmacol Sci 23(21):9436–9444. https://doi.org/10.26355/eurrev_201911_19437
Wang N, Zhang T (2018) Downregulation of microRNA-135 promotes sensitivity of non-small cell lung cancer to gefitinib by targeting TRIM16. Oncol Res 26(7):1005–1014. https://doi.org/10.3727/096504017X15144755633680
Yang W, Feng W, Wu F, Gao Y, Sun Q, Hu N, Lu W, Zhou J (2020) MiR-135-5p inhibits TGF-beta-induced epithelial–mesenchymal transition and metastasis by targeting SMAD3 in breast cancer. J Cancer 11(21):6402–6412. https://doi.org/10.7150/jca.47083
Zhang H (2017) Upregulation of PIM2 by underexpression of microRNA-135–5p improves survival rates of skin allografts by suppressing apoptosis of fibroblast cells. Med Sci Monit 23:107–113. https://doi.org/10.12659/msm.897613
Lytle JR, Yario TA, Steitz JA (2007) Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5’ UTR as in the 3’ UTR. Proc Natl Acad Sci USA 104(23):9667–9672. https://doi.org/10.1073/pnas.0703820104
Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M (2010) SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci 30(16):5754–5766. https://doi.org/10.1523/JNEUROSCI.5007-09.2010
Tang J, Li ZH, Ge SN, Wang W, Mei XP, Wang W, Zhang T, Xu LX, Li JL (2012) The inhibition of spinal astrocytic JAK2-STAT3 pathway activation correlates with the analgesic effects of triptolide in the rat neuropathic pain model. Evid Based Complement Alternat Med 2012:185167. https://doi.org/10.1155/2012/185167
Murray PJ (2007) The JAK-STAT signaling pathway: input and output integration. J Immunol 178(5):2623–2629. https://doi.org/10.4049/jimmunol.178.5.2623
Yoshida Y, Kumar A, Koyama Y, Peng H, Arman A, Boch JA, Auron PE (2004) Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique TRAF6- and p65-dependent mechanism. J Biol Chem 279(3):1768–1776. https://doi.org/10.1074/jbc.M311498200
Huang C, Ma R, Sun S, Wei G, Fang Y, Liu R, Li G (2008) JAK2-STAT3 signaling pathway mediates thrombin-induced proinflammatory actions of microglia in vitro. J Neuroimmunol 204(1–2):118–125. https://doi.org/10.1016/j.jneuroim.2008.07.004
Samavati L, Rastogi R, Du W, Huttemann M, Fite A, Franchi L (2009) STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol 46(8–9):1867–1877. https://doi.org/10.1016/j.molimm.2009.02.018
Acknowledgements
The authors would like to thank those previous researchers who updated their data onto the databases employed in the present study.
Funding
This work is supported by the Fundamental Research Funds for the Central Universities (No. 2042019kf0073).
Author information
Authors and Affiliations
Contributions
M.L., X.F.C., and Z.H.C. designed the experiments. M.L. and X.F.C. collected samples. M.L., X.F.C., and H.Y. conducted the experiments and acquired the data. M.L., H.Y., and J.L.C. analyzed the data. M.L. and X.F.C. wrote the manuscript. H.Y., J.L.C., and C.H.L. revised the manuscript. All authors approved the final version. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental approaches were reviewed and approved by the Institutional Animal Care and Use Committee of the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, M., Cheng, X., Yan, H. et al. MiR-135-5p Alleviates Bone Cancer Pain by Regulating Astrocyte-Mediated Neuroinflammation in Spinal Cord through JAK2/STAT3 Signaling Pathway . Mol Neurobiol 58, 4802–4815 (2021). https://doi.org/10.1007/s12035-021-02458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02458-y