Abstract
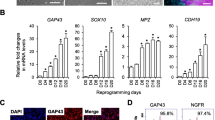
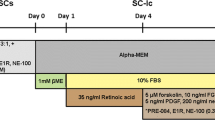
Schwann cells (SCs) are considered potentially attractive candidates for transplantation therapies in neurodegenerative diseases. However, problems arising from the isolation and expansion of the SCs restrict their clinical applications. Establishing an alternative Schwann-like cell type is a prerequisite. Epidermal neural crest stem cells (EPI-NCSCs) are well studied for their autologous accessibility, along with the ability to produce major neural crest derivatives and neurotrophic factors. In the current study, we explored insulin influence, a well-known growth factor, on directing EPI-NCSCs into the Schwann cell (SC) lineage. EPI-NCSCs were isolated from rat hair bulge explants. The viability of cells treated with a range of insulin concentrations (0.05–100 μg/ml) was defined by MTT assay at 24, 48, and 72 h. The gene expression profiles of neurotrophic factors (BDNF, FGF-2, and IL-6), key regulators involved in the development of SC (EGR-1, SOX-10, c-JUN, GFAP, OCT-6, EGR-2, and MBP), and oligodendrocyte (PDGFR-α and NG-2) were quantified 1 and 9 days post-treatment with 0.05 and 5 μg/ml insulin. Furthermore, the protein expression of nestin (stemness marker), SOX-10, PDGFR-α, and MBP was analyzed following the long-term insulin treatment. Insulin downregulated the early-stage SC differentiation marker (EGR-1) and increased neurotrophins (BDNF and IL-6) and pro-myelinating genes, including OCT-6, SOX-10, EGR-2, and MBP, as well as oligodendrocyte differentiation markers, upon exposure for 9 days. Insulin can promote EPI-NCSC differentiation toward SC lineage and possibly oligodendrocytes. Thus, employing insulin might enhance the EPI-NCSCs efficiency in cell transplantation strategies.
Graphical abstract








Similar content being viewed by others
References
Jessen KR, Mirsky R (2005) The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6(9):671–682
Zochodne DW (2008) Neurobiology of peripheral nerve regeneration. Cambridge University Press Cambridge, UK
Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, Xie Y, Miller FD, Tetzlaff W (2007) Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci 27(36):9545–9559
Bunge RP et al (1993) Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 59:75
Wang ZH, Walter GF, Gerhard L (1996) The expression of nerve growth factor receptor on Schwann cells and the effect of these cells on the regeneration of axons in traumatically injured human spinal cord. Acta Neuropathol 91(2):180–184
Hampton DW, Innes N, Merkler D, Zhao C, Franklin RJM, Chandran S (2012) Focal immune-mediated white matter demyelination reveals an age-associated increase in axonal vulnerability and decreased remyelination efficiency. Am J Pathol 180(5):1897–1905
Zawadzka M, Rivers LE, Fancy SPJ, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A et al (2010) CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6(6):578–590
Itoyama Y, Webster HDF, Richardson EP, Trapp BD (1983) Schwann cell remyelination of demyelinated axons in spinal cord multiple sclerosis lesions. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 14(3):339–346
Garcia-Diaz B, Baron-Van Evercooren A (2020) Schwann cells: rescuers of central demyelination. Glia 68:1945–1956
Silva NA, Sousa N, Reis RL, Salgado AJ (2014) From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 114:25–57
Sakaue M, Sieber-Blum M (2015) Human epidermal neural crest stem cells as a source of Schwann cells. Development 142(18):3188–3197
BerrocAl YA et al (2013) Transplantation of Schwann cells in a collagen tube for the repair of large, segmental peripheral nerve defects in rats. J Neurosurg 119(3):720–732
Sieber-Blum M, Schnell L, Grim M, Hu YF, Schneider R, Schwab ME (2006) Characterization of epidermal neural crest stem cell (EPI-NCSC) grafts in the lesioned spinal cord. Mol Cell Neurosci 32(1-2):67–81
Hu YF, Zhang ZJ, Sieber-Blum M (2006) An epidermal neural crest stem cell (EPI-NCSC) molecular signature. Stem Cells 24(12):2692–2702
Hu YF, Gourab K, Wells C, Clewes O, Schmit BD, Sieber-Blum M (2010) Epidermal neural crest stem cell (EPI-NCSC)--mediated recovery of sensory function in a mouse model of spinal cord injury. Stem Cell Rev Rep 6(2):186–198
Narytnyk A, Verdon B, Loughney A, Sweeney M, Clewes O, Taggart MJ, Sieber-Blum M (2014) Differentiation of human epidermal neural crest stem cells (hEPI-NCSC) into virtually homogenous populations of dopaminergic neurons. Stem Cell Rev Rep 10(2):316–326
Clewes O, Narytnyk A, Gillinder KR, Loughney AD, Murdoch AP, Sieber-Blum M (2011) Human epidermal neural crest stem cells (hEPI-NCSC)—characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Rev Rep 7(4):799–814
Manu MS, Rachana KS, Advirao GM (2018) The correlation between insulin and OCT-6 transcription factor in Schwann cells and sciatic nerve of diabetic rats. Genes & diseases 5(2):130–136
Shetter AR, Muttagi G, Sagar CBK (2011) Expression and localization of insulin receptors in dissociated primary cultures of rat Schwann cells. Cell Biol Int 35(3):299–304
Sugimoto K, Murakawa Y, Sima AA (2002) Expression and localization of insulin receptor in rat dorsal root ganglion and spinal cord. J Peripher Nerv Syst 7(1):44–53
Fernyhough P, Diemel LT, Brewster WJ, Tomlinson DR (1994) Deficits in sciatic nerve neuropeptide content coincide with a reduction in target tissue nerve growth factor messenger RNA in streptozotocin-diabetic rats: effects of insulin treatment. Neuroscience 62(2):337–344
Bansal S, Chopra K (2015) Differential role of estrogen receptor modulators in depression-like behavior and memory impairment in rats with postmenopausal diabetes. Menopause 22(10):1117–1124
Liao G-Y, An JJ, Gharami K, Waterhouse EG, Vanevski F, Jones KR, Xu B (2012) Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med 18(4):564–571
Yamashita H, Oh-ishi S, Kizaki T, Nagasawa J, Saitoh D, Ohira Y, Ohno H (1995) Insulin stimulates the expression of basic fibroblast growth factor in rat brown adipocyte primary culture. Eur J Cell Biol 68(1):8–13
Kimura K, Tanida M, Nagata N, Inaba Y, Watanabe H, Nagashimada M, Ota T, Asahara SI et al (2016) Central insulin action activates Kupffer cells by suppressing hepatic vagal activation via the nicotinic alpha 7 acetylcholine receptor. Cell Rep 14(10):2362–2374
Pournajaf S, Valian N, Mohaghegh Shalmani L, Khodabakhsh P, Jorjani M, Dargahi L (2020) Fingolimod increases oligodendrocytes markers expression in epidermal neural crest stem cells. Eur J Pharmacol 885:173502
Pajtler K et al (2010) Production of chick embryo extract for the cultivation of murine neural crest stem cells. JoVE (Journal of Visualized Experiments) 45:e2380
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Topilko P, Levi G, Merlo G, Mantero S, Desmarquet C, Mancardi G, Charnay P (1997) Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J Neurosci Res 50(5):702–712
Jessen K et al (1990) Three markers of adult non-myelin-forming Schwann cells, 217c (Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development 109(1):91–103
Balakrishnan A, Stykel MG, Touahri Y, Stratton JA, Biernaskie J, Schuurmans C (2016) Temporal analysis of gene expression in the murine Schwann cell lineage and the acutely injured postnatal nerve. PLoS One 11(4):e0153256
Boerboom A et al (2017) Molecular mechanisms involved in Schwann cell plasticity. Front Mol Neurosci 10:38
Agis-Balboa RC, Arcos-Diaz D, Wittnam J, Govindarajan N, Blom K, Burkhardt S, Haladyniak U, Agbemenyah HY et al (2011) A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J 30(19):4071–4083
Erickson RI, Paucar AA, Jackson RL, Visnyei K, Kornblum H (2008) Roles of insulin and transferrin in neural progenitor survival and proliferation. J Neurosci Res 86(8):1884–1894
Ziegler AN, Levison SW, Wood TL (2015) Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat Rev Endocrinol 11(3):161–170
Hawryluk GW et al (2012) In vitro characterization of trophic factor expression in neural precursor cells. Stem Cells Dev 21(3):432–447
Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H (1992) Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol 119(1):45–54
Ornitz DM, Itoh N (2001) Fibroblast growth factors. Genome Biol 2(3):1–12
Bolin LM et al (1995) Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J Neurochem 64(2):850–858
Lin G, Zhang H, Sun F, Lu Z, Reed-Maldonado A, Lee YC, Wang G, Banie L et al (2016) Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells. Translational andrology and urology 5(2):167–175
Morgan L, Jessen KR, Mirsky R (1994) Negative regulation of the P0 gene in Schwann cells: suppression of P0 mRNA and protein induction in cultured Schwann cells by FGF2 and TGF beta 1, TGF beta 2 and TGF beta 3. Development 120(6):1399–1409
Lara-Ramirez R et al (2008) Expression of interleukin-6 receptor α in normal and injured rat sciatic nerve. Neuroscience 152(3):601–608
Ko JS, Eddinger KA, Angert M, Chernov AV, Dolkas J, Strongin AY, Yaksh TL, Shubayev VI (2016) Spinal activity of interleukin 6 mediates myelin basic protein-induced allodynia. Brain Behav Immun 56:378–389
Park D, Xiang AP, Mao FF, Zhang L, di CG, Liu XM, Shao Y, Ma BF et al (2010) Nestin is required for the proper self-renewal of neural stem cells. Stem Cells 28(12):2162–2171
Slutsky SG, Kamaraju AK, Levy AM, Chebath J, Revel M (2003) Activation of myelin genes during transdifferentiation from melanoma to glial cell phenotype. J Biol Chem 278(11):8960–8968
Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M (2002) Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev 16(2):165–170
Morino K, Maegawa H, Fujita T, Takahara N, Egawa K, Kashiwagi A, Kikkawa R (2001) Insulin-induced c-Jun N-terminal kinase activation is negatively regulated by protein kinase C δ. Endocrinology 142(6):2669–2676
Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D'Antonio M, Mirsky R, Jessen KR (2004) Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol 164(3):385–394
Sun X et al (2018) Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: potential advantage of cellular transient memory function. Stem Cell Res Ther 9(1):1–20
Gao K, Wang CR, Jiang F, Wong AYK, Su N, Jiang JH, Chai RC, Vatcher G et al (2013) Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia 61(12):2063–2077
Shettar A, Muttagi G (2012) Developmental regulation of insulin receptor gene in sciatic nerves and role of insulin on glycoprotein P0 in the Schwann cells. Peptides 36(1):46–53
Li P, Li HX, Jiang HY, Zhu L, Wu HY, Li JT, Lai JH (2017) Expression of NG2 and platelet-derived growth factor receptor alpha in the developing neonatal rat brain. Neural Regen Res 12(11):1843–1852
Acknowledgements
The authors thank Dr. Sareh Pandamooz for her kind advice in cell isolation and characterization. The graphical abstract was created with Biorender.com.
Availability of Data and Materials
The datasets used and/or analyzed during this study are available from the corresponding author on request.
Funding
This study was funded by Research Affairs (Grant No. 15678) of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Pariya Khodabakhsh: formal analysis, investigation, and writing — original draft. Safura Pournajaf: investigation and writing — review and editing. Leila Mohaghegh Shalmani: investigation. Abolhassan Ahmadiani: conceptualization and supervision. Leila Dargahi: conceptualization, methodology, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All experimental protocols of this study were approved by the Ethical Committee of Neuroscience Research Center, Shahid Beheshti University of Medical Sciences (ethics approval code: IR.SBMU.PHNS.REC.1397.024) in compliance with the standards of the European Communities Council Directive (86/609/EEC). All efforts were made to reduce animal suffering and the number of mice needed for the study.
Consent for Publication
All authors provided a final review and approved the manuscript before submission. The article is original, has not already been published in a journal, and is not currently under consideration by another journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figure S1.
EPI-NCSC viability after exposure to the regular culture medium and different concentrations of insulin for 6, 24, and 72 hours (A, B, and C respectively). The assay indicated the absence of any significant decrease in viability of cells treated with insulin at concentrations less than 100 μg/mL following 24, 48, and 72h compared to the control group. Insulin at 5 μg/mL induced a marked increase in cell viability after 72h. Values are presented as the mean ± SEM. *P<0.05, **P<0.01 compared to control group at 72h (n=6). (PNG 2334 kb)
Rights and permissions
About this article
Cite this article
Khodabakhsh, P., Pournajaf, S., Mohaghegh Shalmani, L. et al. Insulin Promotes Schwann-Like Cell Differentiation of Rat Epidermal Neural Crest Stem Cells. Mol Neurobiol 58, 5327–5337 (2021). https://doi.org/10.1007/s12035-021-02423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02423-9